Study explores how brain disruption may foster schizophrenia

(Medical Xpress)—A team led by Yale researchers has used pharmacological neuroimaging and computational modeling to examine large-scale functional organization in the human brain. Their novel approach has yielded important insights about how disruption of a specific molecular signaling mechanism within neural systems may contribute to symptoms of schizophrenia. The results are reported online ahead of print in the Proceedings of the National Academy of Sciences.
Previous studies on this topic have been limited to processes in local circuits; however, cognition involves large-scale brain systems with multiple interacting regions. The current study suggests that coordination of these large-scale systems depends on the proper functioning of glutamate – a key excitatory neurotransmitter.
"While neuroimaging alone has aided our understanding of higher cognitive function, it cannot reveal cellular-level mechanisms in humans. The addition of pharmacology and computational modeling help us start to see a more complete picture," said Alan Anticevic, PhD, associate research scientist in psychiatry at the Yale School of Medicine and lead author of the study. "This deeper understanding could lead to better treatment of neuropsychiatric conditions such as schizophrenia."
The team found that a balance of excitatory and inhibitory function in the human brain is vital for optimal large-scale network coordination and cognition, and that inhibitory neurons play a crucial role in producing the behavioral deficits that may occur in individuals with schizophrenia.
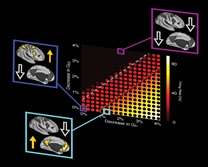
Additionally, for the first time, the team elucidated the link between drug effects and behavior using a mathematical model that was built from the level of cells. Perturbing the balance of neuronal inhibition inside the model closely matched experimental observations.
Philip Corlett, PhD, assistant professor of psychiatry, and John Krystal, MD, Robert L. McNeil Jr. Professor of Translational Research and chair of psychiatry, share senior authorship. The team's state-of-the-art mathematical models were developed by John Murray, a PhD student in physics at Yale, and Xiao-Jing Wang, PhD, professor of neurobiology, of psychology, and of physics at Yale, both of whom are co-authors of the study.
"These results provide the exciting possibility of understanding psychiatric symptoms at the levels of individual cells, neural systems, and human behavior," said Corlett. Krystal added, "Such translational approaches ultimately offer the promise for rationally-devised treatments for psychiatric conditions."
More information: www.pnas.org/content/early/201 … /1208494109.abstract