New test could help diagnose Alzheimer's disease in live patients
The patient turned 40 over the summer and was already having symptoms that made her neurologist wonder whether she had Alzheimer's disease, the deadly, mind-killing dementia that usually attacks far older people.
She and her husband went to the Adler Institute for Advanced Imaging in Jenkintown, Pa., on a recent morning after 70 tests over the last year failed to explain her worsening symptoms. She was going to try yet another: a newly approved test developed by a Philadelphia biotech firm.
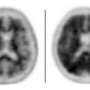
It uses a PET scanner and a radioactive tracer - Amyvid - that binds to amyloid, one of the hallmark proteins that accumulate in the brains of Alzheimer's patients. Until recently, the only sure way to know whether people had Alzheimer's was to examine their brains after death.
The Food and Drug Administration approved the test in April. It is both a sign of the future of dementia treatment - enabling doctors to identify people at risk for a dread disease even before they have symptoms - and an example of the challenging economic and medical issues new technology so often raises.
The test is costly and possibly confusing. There is now no good, long-term Alzheimer's treatment. The diagnosis could subject patients to discrimination by employers and insurers, let alone intense anxiety.
Claudia Kawas, a geriatric neurologist at the University of California, Irvine, said a patient strode into her office soon after the FDA approved the procedure and asked for the "Alzheimer's test." In one hand, he held an article about the approval of Amyvid. In the other, he had a document from a right-to-die group. If the test was positive, he told her, he would get his affairs in order and kill himself within 60 days.
Although she thinks the test can be useful, she was glad her center didn't have it. She says she wouldn't have it done on herself, though she's noticing some memory changes. "The information, I think, wouldn't help me in any way," she said.
The Adler patient, who asked that her name not be used, is a former college English major who is having trouble finding words, thinking clearly and understanding even slightly complicated reading material. On a recent shopping excursion, her sister found her wandering aimlessly. She's having memory, spatial and balance problems, and has given up driving.
Alzheimer's would be unusual at her age, but an earlier PET scan showed a pattern suggestive of the disease. The new test would support that diagnosis or rule it out.
The patient and her husband, who met on her 21st birthday and became engaged six weeks later, want to know what to tell their children, how to face their future, however bleak it may be. Still, the woman hoped their search for a diagnosis would not end with this test. "Alzheimer's at this age is a pretty scary endeavor."
Radiologist Lee Adler told her the test, which combines a CT and PET scan, would take about 23 minutes. She had to lie perfectly still on a table, her head immobile between two wedges. It would be quiet.
Adler, who began doing the test as part of research in 2007, told her the absence of amyloid would mean she didn't have Alzheimer's. Its presence is more complicated. Some people with amyloid in their brains think normally. Some may be at higher risk for Alzheimer's, but it's too soon to know whether amyloid is a sign of what experts are calling "preclinical" disease.
Patients with mild cognitive impairment, often a precursor to dementia, along with amyloid deposits may be at higher risk. "We can exclude the diagnosis," Adler said, "but not make it."
The woman climbed onto the scanner and waited for the test to begin.
Rajan Agarwal, an Abington Memorial Hospital radiologist, did the country's first three commercial Amyvid scans in June and has had no referrals since. Adler also has done three. Though doctors think the test can be a blockbuster, PETNET, a Siemens subsidiary that distributes the tracer, said it had made 100 commercial doses for this region since June.
The tracer is made individually for each patient on test day at 15 sites around the country, then delivered within a 2{-hour radius. In this region, it's mixed in North Wales.
Lack of coverage for the $3,000 test is the biggest barrier to it here. Medicare's decision on coverage, which often sets the standard for private insurers, is due by July.
Doctors don't know what causes Alzheimer's. Amyloid and tau in the brain are the proteins that define the diagnosis, but it's still unclear whether amyloid causes symptoms or defends against the disease. Drugs that target it have been disappointing so far.
Doctors say Amyvid is likely to be most useful when people have symptoms but the diagnosis is uncertain. It would help doctors decide between, say, Alzheimer's and frontotemporal dementia. Negative Amyvid results can spur doctors to seek other explanations and avoid prescribing useless drugs that have side effects.
Recent studies involving Amyvid, which is made by Avid Radiopharmaceuticals, an Eli Lilly & Co. subsidiary, have shown that a surprising number of patients thought to have Alzheimer's didn't. A paper in the October issue of Annals of Neurology noted that 12 of 53 patients diagnosed with Alzheimer's by expert physicians had negative PET scans for amyloid.
One emerging lesson, said Jason Karlawish, an Alzheimer's expert at the University of Pennsylvania, is that Alzheimer's may be more than one disease or variant.
In an article published in the fall in the journal Alzheimer's & Dementia, a group of international experts who gathered at Penn over the summer said other biomarkers were being tested in clinical trials, from imaging compounds to analyses of blood and cerebrospinal fluid.
Laws prohibiting the use of biomarkers in insurance or employment decisions may also be in order until their clinical value is fully understood, they said.
For now, one of the most obvious benefits of biomarkers is that they can help researchers put patients in the right clinical trials. That raises the odds that a drug meant to treat Alzheimer's is being tested on people with the disease.
Dementia experts know changes associated with Alzheimer's begin decades before it's diagnosed. When there is a treatment, it almost certainly will make sense to start early, before the disease causes extensive brain damage. Then, demand for tests like Amyvid will skyrocket.
"The race is on here between the biomarkers and the therapeutics," said Rise Sperling, a Harvard University Alzheimer's expert.
In Jenkintown, Adler's patient emerged from her scan. "It was lovely," she said.
Adler told the couple he could see that the woman's brain had not atrophied. Good news.
Later, the patient, who did not want her name used until she knows what's wrong with her, learned the test had been negative. She's left with the mystery, but glad to rule out Alzheimer's.
"It's actually a relief," she said, "because that would have been the worst-case scenario."
(c)2012 The Philadelphia Inquirer
Distributed by MCT Information Services