Implantable telescope lens to treat macular degeneration
(Medical Xpress)—Retired entrepreneur Willis "James" Hindman, 77, always enjoyed raising and watching thoroughbred race horses run on his farm in Westminster, Md. "There is nothing more beautiful than seeing a horse in motion and at full speed. It's something very special to me," says Hindman.
But when severe age-related macular degeneration (AMD) destroyed Hindman's vision to the point where he couldn't read, see faces of family and friends or watch his horses, he says he became depressed and a "captive of my limitations."
Hindman, founder and former CEO of Jiffy Lube International, is one of the approximately two million Americans who have the advanced form of AMD, which affects the region of the retina responsible for central, detailed vision, and is the leading cause of irreversible vision loss and legal blindness in people over the age of 65.
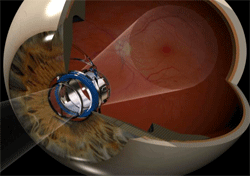
Now, a relatively new device, essentially an implantable telescope, is available to people like Hindman, who underwent his implantation in December 2012, is offering hope for those "aging eyes." The U.S. Food and Drug Administration approved the Implantable Miniature Telescope (IMT), which works like the telephoto lens of a camera, in 2010.
The surgical procedure involves removing the eye's natural lens, as with cataract surgery, and replacing the lens with the IMT. The tiny telescope is implanted behind the iris, the colored, muscular ring around the pupil.
"While it doesn't cure AMD, it will help improve the vision of patients, like Mr. Hindman, and help them resume their favorite activities and independence," said Oliver D. Schein, M.D., M.P.H., M.B.A., Burton E. Grossman Professor of Ophthalmology and director of the Comprehensive Eye Service at The Wilmer Eye Institute at Johns Hopkins.
The Wilmer Eye Institute is the first place in Maryland to implant the IMT since its approval by the FDA. Schein, who performed the surgery on Hindman, led the Wilmer Eye Institute's participation in a four-year, multi-center FDA study that provided long-term data on the efficacy of the IMT in patients with end stage age AMD.
Hindman is one of three (two from the trial, one post-FDA approval) Wilmer patients in Maryland to have the telescope implanted to date. The device itself costs approximately $15,000, which does not include the cost of surgery and rehabilitation. However, Schein says that the IMT is covered by Medicare for eligible patients. Schein says that after the IMT implantation, patients participate in an extensive rehabilitation program that involves training them to effectively use the device. Rehabilitation postsurgery takes about six months to a year.
Hindman, who is currently receiving therapy at Wilmer's Low Vision Rehabilitative Services from a visual rehabilitation specialist, is still learning how to use his enhanced eye.
"After surgery, the therapy is the most critical component. You need months of training in helping to reteach the brain to learn how to use each eye differently for a specific task," explains Judith Goldstein, O.D., FAAO, assistant professor of ophthalmology and rehabilitative medicine and chief of the Low Vision and Visual Rehabilitation Service at the Wilmer Eye Institute at Johns Hopkins. "The telescope eye sees things centrally and the non-telescope eye basically sees things peripherally. It's unlike anything they've ever experienced."
Hindman says the change in his life has been dramatic over the past four months.
"The services I have been provided at Johns Hopkins have just been tremendous. They've been inspiring. They make me want to get up in the morning," says Hindman. "I have started becoming more interested in working with my horses because I can see."
Schein cautions though that the surgery is not for everyone and in keeping with FDA guidelines potential candidates must be 75 years of age or older, have irreversible dry AMD and no longer be a candidate for drug treatment. The guidelines also exclude those who have had previous cataract surgery in the eye to be implanted.
The IMT is part of VisionCare Ophthalmic Technologies CentraSight treatment program. Dr. Schein is a paid research consultant for VisionCare.