Study offers clues to making vaccine for infant respiratory illness
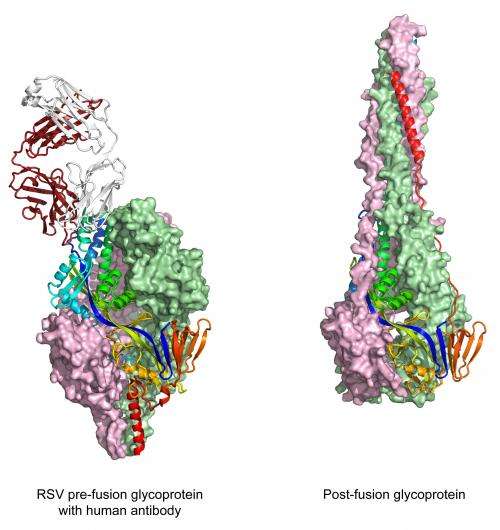
An atomic-level snapshot of a respiratory syncytial virus (RSV) protein bound to a human antibody represents a leap toward developing a vaccine for a common—and sometimes very serious—childhood disease. The findings, by scientists from the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, define the vulnerable shape of a critical RSV component called the fusion glycoprotein.
The NIAID scientists determined the fusion glycoprotein's shape as it appears before its interaction with human cells. It is this pre-fusion shape that is most vulnerable to neutralizing antibodies. Progress toward an RSV vaccine has been stalled in part because researchers did not previously know about a highly vulnerable site at the tip of the pre-fusion form of the fusion glycoprotein. Now that the structure has been solved and the site of antibody vulnerability revealed, scientists can use the new structural information to design vaccines capable of eliciting potent antibodies aimed at the target on top of the pre-fusion state of the glycoprotein.
Almost everyone is infected with RSV before turning three years of age. Most children recover quickly from such symptoms as sneezing, runny nose and cough, but the virus is a leading cause of hospitalization in children under age one. In the United States each year between 75,000 and 125,000 children in this age group are hospitalized with RSV infection. Globally, RSV infection accounts for nearly 7percent of deaths among children between the age of one month and one year. The only drug available to prevent severe RSV illness is a monoclonal antibody, palivizumab, which binds to the RSV fusion glycoprotein. In their study, the NIAID researchers showed how three antibodies that potently neutralize RSV all bind to the newly revealed site on the fusion glycoprotein of RSV. Thus, in addition to new clues for vaccine developers, the NIAID findings also provide a structural basis for how these antibodies neutralize RSV. This insight could accelerate development of these antibodies into therapies to treat or prevent severe RSV disease in very young infants, who are the most vulnerable to serious illness.
More information: JS McLellan et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science DOI: 10.1126/science.1234914 (2013).