Toward broad-spectrum antiviral drugs for common cold and other infections
Scientists are reporting progress in the search for the first broad-spectrum drugs to combat human rhinoviruses (HRVs), which cause humanity's most common infectious diseases. Their study on these potential drugs for infections that include the common cold appears in the journal ACS Medicinal Chemistry Letters.
Angus MacLeod and colleagues note that although many HRV infections cause mild disease, they can lead to dangerous complications for millions of people with asthma and chronic obstructive pulmonary disease. Previous potential drugs for HRV either didn't work or caused unacceptable side effects, leaving only one potential drug still under development in clinical trials. MacLeod's team set out to find new antiviral candidates to meet this serious health challenge.
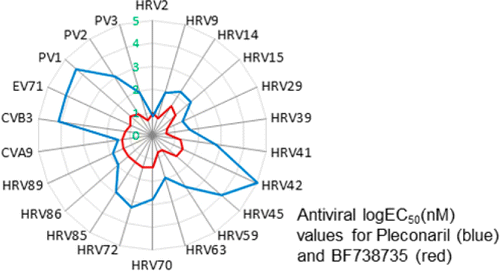
They describe identifying and successfully testing a group of compounds that work against human rhinovirus, Coxsackie virus, poliovirus and enterovirus-71—the cause of hand, foot and mouth disease. The substances work by blocking the ability of these viruses to multiply.
More information: "Identification of a Series of Compounds with Potent Antiviral Activity for the Treatment of Enterovirus Infections" ACS Med. Chem. Lett., Article ASAP. DOI: 10.1021/ml400095m