August 22, 2013 report
Team learns how sleeping sickness parasite defeats immune system
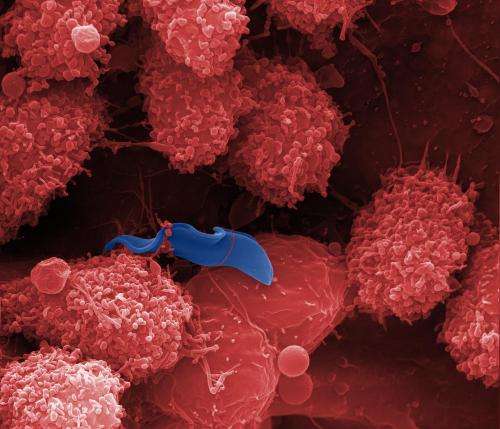
(Medical Xpress)—A team of researchers with members from across Europe has discovered the mechanism by which the sleeping sickness parasite overcomes the immune system in humans. In their paper published in the journal Nature, the team describes the three step process that the parasite uses to defeat an immune system response. They also report that they have developed a mutant type of protein that disrupts the parasitic process allowing the immune system to destroy the invader.
Sleeping sickness is caused by a parasite which is usually transmitted by the tsetse fly—7,197 new cases were reported in 2012 alone. It's constrained mainly to Africa and no known vaccine exists to prevent it. In many cases, people who are infected live with it for many years, eventually succumbing to its debilitating effects (headaches, fever, itching, joint pain and eventually swollen lymph nodes and other organ damage as well as brain injury). The parasite gains entry to the body when a victim is bitten then travels to other parts of the body via the bloodstream. Modern treatments for the disease have reduced deaths dramatically, but the hope is that a vaccine can be created that will prevent the misery it inflicts.
The researchers have been studying the gambiense strain of the sleeping sickness parasite which is responsible for the majority (97 percent) of human deaths. In so doing they have learned that the parasites use a three step process to outwit the immune system response.
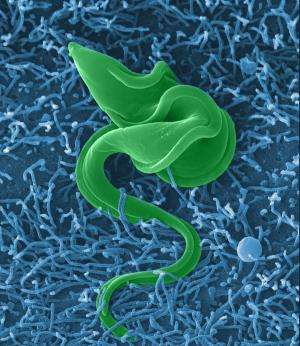
In the first stage, the parasite creates a protein to stiffen its membranes, making it difficult for the immune system protein apoL1 to enter the parasite body and kill it. The second stage involves building up its inner defenses to make it even more difficult for apol1 to make its way inside. The third stage involves actually digesting the apoL1 protein if it does make its way inside the parasite, preventing its absorption which would kill it.
Because the team was able to identify the process by which the parasite foils the immune system, they were able to develop a mutant strain of the apoL1 protein which was not fooled by the tactics of the parasite and was therefore able to kill it. A lot more research will have to be conducted on the mutant strain of course, to make sure it doesn't behave in unexpected ways, before human trials can begin.
More information: Mechanism of Trypanosoma brucei gambiense resistance to human serum, Nature (2013) DOI: 10.1038/nature12516
Abstract
The African parasite Trypanosoma brucei gambiense accounts for 97% of human sleeping sickness cases. T. b. gambiense resists the specific human innate immunity acting against several other tsetse-fly-transmitted trypanosome species such as T. b. brucei, the causative agent of nagana disease in cattle. Human immunity to some African trypanosomes is due to two serum complexes designated trypanolytic factors (TLF-1 and -2), which both contain haptoglobin-related protein (HPR) and apolipoprotein LI (APOL1)2, 3, 4. Whereas HPR association with haemoglobin (Hb) allows TLF-1 binding and uptake via the trypanosome receptor TbHpHbR, TLF-2 enters trypanosomes independently of TbHpHbR. APOL1 kills trypanosomes after insertion into endosomal/lysosomal membranes. Here we report that T. b. gambiense resists TLFs via a hydrophobic ?-sheet of the T. b. gambiense-specific glycoprotein (TgsGP)8, which prevents APOL1 toxicity and induces stiffening of membranes upon interaction with lipids. Two additional features contribute to resistance to TLFs: reduction of sensitivity to APOL1 requiring cysteine protease activity, and TbHpHbR inactivation due to a L210S substitution. According to such a multifactorial defence mechanism, transgenic expression of T. b. brucei TbHpHbR in T. b. gambiense did not cause parasite lysis in normal human serum. However, these transgenic parasites were killed in hypohaptoglobinaemic serum, after high TLF-1 uptake in the absence of haptoglobin (Hp) that competes for Hb and receptor binding. TbHpHbR inactivation preventing high APOL1 loading in hypohaptoglobinaemic serum may have evolved because of the overlapping endemic area of T. b. gambiense infection and malaria, the main cause of haemolysis-induced hypohaptoglobinaemia in western and central Africa.
© 2013 Medical Xpress

















