Researchers develop model to study immune response to infections that cause peptic ulcers
(Medical Xpress)—Researchers at the Virginia Bioinformatics Institute have developed a new large animal model to study how the immune system interacts with the stomach bacterium Helicobacter pylori, the leading cause of peptic ulcer disease.
The discovery in the October edition of the journal Infection and Immunity may inform changes in the ways doctors treat patients. An estimated 4 million Americans have sores in the stomach lining known as peptic ulcers, according to the American Gastroenterological Association.
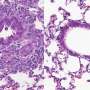
Although the bacterium is found in more than half the world's population, most people do not develop diseases. However, some experience chronic inflammation of the stomach, or gastritis, which can lead to the development of ulcers or cancer.
In addition to its role as a pathogen, the bacteria have beneficial effects, preventing certain chronic inflammatory and metabolic diseases, including Type 2 diabetes, and obesity.
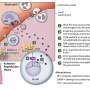
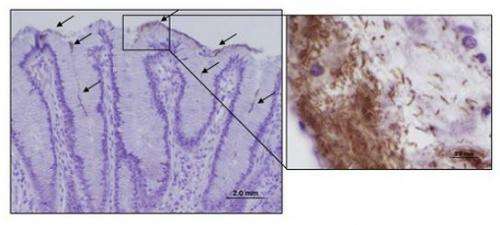
When bacteria reside within host cells, the immune system typically recruits a type of white blood cell called T cells—in this case, CD8+ cytotoxic T cells—to destroy the infected cells.
However, the researchers found that these cells may contribute to tissue damage.
In patients with H. pylori-associated gastritis, higher numbers of cytotoxic T cells are present, indicating that these cells may contribute to the development of gastric lesions.
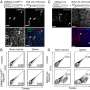
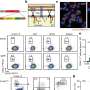
To study immune responses in H. pylori-mediated disease, researchers at the Virginia Bioinformatics Institute's Nutritional Immunology and Molecular Medicine Laboratory developed a pig model that closely mimics the human gastric environment. When pigs were infected with H. pylori, the researchers observed an increase in another type of immune cells called pro-inflammatory CD4+ T helper cells, followed by an increase in CD8+ cytotoxic T cells, according to the study.
Scientists did not observe an increase in CD8+ T cells in mouse and gerbil models of H. pylori infection. However, the rise of the cells in pigs mirrors the recent findings in human clinical studies.
"Pigs have greater anatomic, physiologic and immunologic similarities to humans than mice, the main animal model used in biomedical research," said Raquel Hontecillas, co-director of the Nutritional Immunology and Molecular Medicine Laboratory and the Center for Modeling Immunity to Enteric Pathogens. "The results from our new pig model closely mimic what has been reported in clinical settings, which will allow us to comprehensively and systematically investigate human immune responses to H. pylori."
The discovery will help scientists better understand the complex interactions of H. pylori and its host.
Researchers within the Center for Modeling Immunity to Enteric Pathogens are using results from the pig model and other experimental data to develop a computational model of H. pylori infection. Such modeling efforts aim to develop faster, more efficient ways to predict initiation, progression and outcomes of infection.
More information: iai.asm.org/content/81/10/3803.abstract