Drug pair cuts children's urinary infections up to 80 percent
Long-term use of a drug combination reduces the risk of recurrent urinary tract infection by up to 80 percent in children with the urinary condition vesicoureteral reflux compared to placebo, according to research funded by the National Institutes of Health. Results were published online May 4 in the New England Journal of Medicine to coincide with presentation at the Pediatric Academic Societies Annual Meeting in Vancouver, British Columbia.
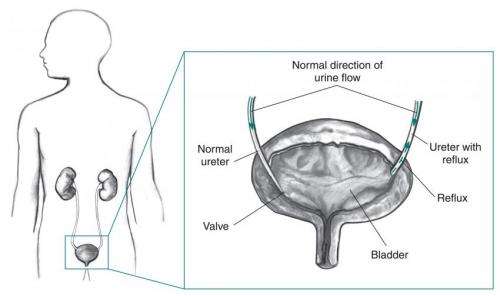
In vesicoureteral reflux (VUR), developmental abnormalities in one or both ureters—tubes connecting the kidneys with the bladder—allow urine to flow back from the bladder into the ureters, and sometimes into the kidneys. VUR is found in 30-40 percent of children who have had a UTI, and is one of the most common urinary tract problems in children.
The risk of recurrent infection was cut by 50 percent in children with VUR using the drug combination trimethoprim/sulfamethoxazole (TMP/SMZ). Children with VUR and bladder and bowel dysfunction saw the greatest reduction, up to an 80 percent lower risk of recurrent infections. This group is also more likely to have recurrent urinary infections, which can increase the risk of kidney scarring and the potential for kidney failure.
For decades, doctors have treated children who have VUR with a small daily dose of TMP/SMZ – often for years, in hopes of preventing recurrent UTIs and kidney damage. The Randomized Intervention for Children with Vesicoureteral Reflux (RIVUR) trial provides the first conclusive clinical evidence for the effectiveness of this practice. It studied a well-defined population of children compared to earlier studies, included a placebo group, used digital imaging, and showed a strong response to treatment.
"We are looking deeper into the data from the RIVUR trial to gain further insight into other factors that may reduce susceptibility to recurrent infections and scarring," said Dr. Marva Moxey-Mims, a pediatric kidney specialist at the NIH's National Institute of Diabetes and Digestive and Kidney Diseases, the study's primary funder. "In the meantime, we can buy children some time with fewer infections, allowing many of them to outgrow reflux as their bodies develop and mature."
While TMP/SMZ significantly reduced recurrent infections, the number of children who developed kidney scarring did not drop. The researchers suggest this may be due to parents' heightened vigilance for UTI symptoms and early treatment in the trial and that most of the children were enrolled after their first infection rather than after multiple infections, when more scarring might occur.
"We also saw some increased antimicrobial resistance, which researchers are looking at more closely," Moxey-Mims said. "However, until we have those results, the use of these drugs appears to provide more benefit than risk in these children."
RIVUR was a two-year clinical trial that randomized 607 children to receive the TMP/SMP drug combination or placebo. The study was conducted in 19 locations across the United States and coordinated by the University of North Carolina at Chapel Hill. General information about clinical studies in children can be found at http://www.nhlbi.nih.gov/childrenandclinicalstudies.













