Researchers find new mechanism for neurodegeneration
A research team led by Jackson Laboratory Professor and Howard Hughes Investigator Susan Ackerman, Ph.D., have pinpointed a surprising mechanism behind neurodegeneration in mice, one that involves a defect in a key component of the cellular machinery that makes proteins, known as transfer RNA or tRNA.
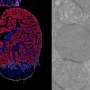
The researchers report in the journal Science that a mutation in a gene that produces tRNAs operating only in the central nervous system results in a "stalling" or pausing of the protein production process in the neuronal ribosomes. When another protein the researchers identified, GTPBP2, is also missing, neurodegeneration results.
"Our study demonstrates that individual tRNA genes can be tissue-specifically expressed in vertebrates," Ackerman says, "and mutations in such genes may cause disease or modify other phenotypes. This is a new area to look for disease mechanisms."
Neurodegeneration—the process through which mature neurons decay and ultimately die—is poorly understood, yet it underlies major human diseases, such as Alzheimer's disease, Parkinson's disease, Huntington's disease and ALS (amyotrophic lateral sclerosis, also known as Lou Gehrig's disease).
While the causes of neurodegeneration are still coming to light, there is mounting evidence that neurons are exquisitely sensitive—much more so than other types of cells—to disruptions in how proteins are made and how they fold.
tRNAs are critical in translating the genetic code into proteins, the workhorses of the cell. tRNAs possess a characteristic cloverleaf shape with two distinct "business" ends—one that reads out the genetic code in three-letter increments (or triplets), and another that transports the protein building block specified by each triplet (known as an amino acid).
In higher organisms, tRNAs are strikingly diverse. For example, while there are 61 distinct triplets that are recognized by tRNAs in humans, the human genome contains roughly 500 tRNA genes. To date little is known about why they are so numerous, whether they carry out overlapping or redundant functions, or whether they possibly have roles beyond the making of proteins.
"Multiple genes encode almost all tRNA types," Ackerman says. "In fact, AGA codons are decoded by five tRNAs in mice. Until now, this apparent redundancy has caused us to completely overlook the disease-causing potential of mutations in tRNAs, as well as other repetitive genes."
Ackerman and her colleagues at The Jackson Laboratory in Bar Harbor, Maine, and Farmington, Conn., The Scripps Research Institute in LaJolla, Calif., and Kumamoto University in Japan pinpointed a mutation in the tRNA gene n-Tr20 as a genetic culprit behind the neurodegeneration observed in mice lacking GTPBP2.
Remarkably, the tRNA's activity is confined to the brain and other parts of the central nervous system, in both mice and humans. The tRNA encoded by n-Tr20 recognizes the triplet code, AGA (which specifies the amino acid arginine).
The n-Tr20 defect disrupts how proteins are made. Specifically, it causes the "factories" responsible for synthesizing proteins, called ribosomes, to stall when they encounter an AGA triplet.
Such stalling can be largely overcome, thanks to the work of a partner protein called GTPBP2. But when this partner is missing—as it is in the mutant mice that Ackerman and her colleagues studied—the stalling intensifies. This is thought to be a driving force behind the neurodegeneration seen in these mice.
More information: Ishimura et al.: Ribosome stalling induced by a mutation of a CNS-specific tRNA causes neurodegeneration. Science, July 24, 2014.