Stem cells may ease urinary incontinence, study says

(HealthDay)—For the millions of women who can't cough, sneeze or laugh without losing bladder control, researchers are testing a treatment that uses stem cells to regenerate weakened urethra muscles.
In a small pilot study, European researchers found that injecting stem cells isolated from patients' own fat tissue improved or eliminated stress incontinence in all participants within a year.
Stress incontinence affects about twice as many women as men because of pelvic floor strain from pregnancy and childbirth.
Most women who choose to treat the condition undergo a procedure that inserts surgical mesh between the urethra and vagina to reduce urine leakage, urologists said. But widening controversy over the use of surgical mesh makes the idea of stem cell treatment even more attractive.
"This is an application that makes sense because of the ease of access to the urethra, which isn't a difficult area to inject," said Dr. Timothy Boone, chairman of urology at Houston Methodist Hospital in Texas, who wasn't involved in the study.
Globally, similar research is under way on the use of stem cells to treat stress incontinence.
However, "a lot of other stem cell therapies are a lot more invasive," Boone added. "It's too soon to tell, but the hope would be that a significant number of women would benefit from this and avoid the possible complications of surgery."
The study is published online in the July issue of the journal Stem Cells Translational Medicine.
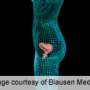
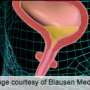
Stress incontinence occurs when pelvic floor muscles supporting the bladder and urethra become too weak to prevent urine flow when pressure is placed on the abdomen. The problem can range from being a nuisance to highly debilitating.
Stem cells, which can develop into many possible cells or organs, are an area of intense study by scientists interested in managing or curing a wide array of serious health conditions.
For this study, researchers from Finland and the Netherlands treated five women suffering from stress incontinence with adipose (fat-derived) stem cells combined with bovine collagen gel—a bulking agent—and saline solution. The women either didn't want to undergo the mesh procedure, known as midurethral sling surgery, or had undergone unsuccessful mesh implants.
Prior to treatment, their stem cells were expanded in a lab for three weeks, then injected in the participants' urethras. After six months, one out of five patients displayed a negative "cough test" with a full bladder, meaning no urine leakage occurred.
At one year, the cough test produced no urine leakage in three of the five patients, and the other two were sufficiently satisfied to decide to end their treatment.
"This is a perfect condition for this type of stem cell technology," said Dr. Elizabeth Kavaler, a urologist at Lenox Hill Hospital in New York City. "You're basically regenerating a very focused, small part of the body, and it's very easy to monitor and see if it's working or not."
Boone and Kavaler said it's premature to speculate on the possible cost of stem cell treatment for urinary incontinence. But Kavaler noted that the ongoing daily costs of managing the condition—often including the purchase of incontinence pads—is "enormous" and that the cost of mesh surgery is at least several thousand dollars.
"I think [stem cell treatment] is a really great idea, and it's just the right part of the body to do this," she said.
More information: The U.S. National Library of Medicine has more about stress urinary incontinence.
Copyright © 2014 HealthDay. All rights reserved.