Intravenous glyburide treatment may reduce dangerous brain swelling after stroke
A phase 2 clinical trial of a drug that may alleviate brain swelling—a dangerous stroke complication—suggests the treatment may help reduce brain injury and death, and information from the study will help design the phase 3 trial. While the trial did not meet its prespecified primary objective, as described in a paper receiving online release in The Lancet Neurology, it did provide additional evidence that intravenous glyburide treatment may improve patient outcomes.
"Although a follow-up clinical study is needed to confirm our preliminary finding, this is the first time a drug has been tested to prevent brain swelling after a major stroke," says W. Taylor Kimberly, MD, PhD, of the Massachusetts General Hospital (MGH) Department of Neurology, co-corresponding author of the report. "We believe that glyburide treatment may be an important strategy to minimize injury resulting from brain swelling."
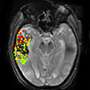
Strokes caused by blockage of the blood supply to a large area of the brain can lead to the accumulation of fluids called edema, causing brain tissue to swell. Since the brain is confined with the skull, significant swelling and the resulting increased pressure can push brain structures out of their normal position, leading to a 50 percent mortality rate for this complication. Treatment with drugs designed to draw fluids out of the brain have had limited effectiveness, and hemicraniectomy—surgical removal of a portion of the skull to allow brain tissue to swell freely—is not appropriate for all patients and carries its own risk of complications.
Previous studies in animal models of stroke have suggested that glyburide—a drug used to treat type 2 diabetes - might prevent brain swelling in stroke patients. A pilot study led by Kimberly and his co-corresponding author Kevin Sheth, MD, Department of Neurology, Yale University School of Medicine, indicated that glyburide treatment was safe for stroke patients and led to the phase 2 trial, sponsored by Remedy Pharmaceuticals. Conducted at 18 hospitals across the U.S., the study - entitled the Glyburide Advantage in Malignant Edema and Stoke (GAMES-RP) trial—enrolled 77 patients with strokes affecting one-third or more of a brain hemisphere. Participants were randomly assigned to continuous intravenous treatment with either glyburide or a placebo for a total of 72 hours.
The prespecified primary endpoint of the study was the proportion of patients who, 90 days after their stroke, achieved scores of 0 to 4 on a standardized stroke scale—a range extending from no symptoms to moderately severe disability - without surgery to relieve brain swelling. While around 40 percent of those in each group met those criteria, the authors note that the decision to have surgery was made by the physicians caring for each patient and may have been arrived at more often at some sites than at others.
"Deciding to proceed to a hemicraniectomy is complicated and depends on the judgment of the stroke neurologist, of the neurosurgeon, and the wishes of patients or their families," says Sheth. "This led to variation in practice across the sites, which is appropriate for clinical care but not ideal for the endpoint of a clinical trial; and we believe this is the main reason the primary endpoint was not reached."
The results for other endpoints, however, were more promising - particularly the brain imaging measure of midline shift, which reflects the extent to which brain structures are pressed out of place by brain swelling. "The degree of midline shift was reduced about 40 percent in patients treated with glyburide, and there was a similar reduction in levels of MMP-9, a biomarker that previous studies have associated with brain swelling after stroke," says Kimberly. "From a clinical standpoint, there was a 50 percent reduction in mortality and a promising trend towards better functional outcome at 90 days." The results of this study are being used to design the phase 3 trial, which will begin enrolling patients in 2017.
More information: The Lancet Neurology, DOI: 10.1016/S1474-4422