New strategy to obtain a specific type of amyloid-beta aggregate that may underlie neuronal death in Alzheimer's disease
The brains of millions of people suffering from Alzheimer's disease are slowly and inescapably being depleted of neurons, but the cause of neuronal death is still unknown. Several studies propose that the interaction of the amyloid-beta protein with the neuronal membrane causes neurotoxicity.
Today, a study headed by associate researcher Natàlia Carulla at the Institute for Research in Biomedicine (IRB Barcelona), in collaboration with scientists at the University of Leuven (Belgium), the University of Groningen (the Netherlands), and the University of Barcelona, describes for the first time a strategy to obtain a membrane-associated amyloid-beta aggregate that adopts a specific barrel-like structure. This type of structure, which is present in other proteins found in nature, has the capacity to form pores in cell membranes. Interpreted in the context of Alzheimer's disease, this discovery suggests that the aggregate can also perforate the membrane of neurons, alter the equilibrium of these cells, and trigger their death.
The description of the barrel-shaped aggregates in the journal Proceedings of the National Academy of Sciences (PNAS) is the first step to establishing the involvement of this aggregate in Alzheimer's disease. Confirming the involvement would validate it as a new therapeutic target for this condition. The scientists made this discovery in the lab by studying amyloid-beta protein in simple membrane model systems.
Attack to the amyloid-beta protein
Dr. Carulla developed methods to identify the nature and structure of the toxic amyloid-beta species. "Everyone talks about the relevance of oligomers (aggregates made of the same protein unit) of amyloid-beta and their contribution to Alzheimer's disease. But if we don't know which structural features characterize them, we won't know what to look for, and therefore we won't be able to demonstrate or disprove anything," explains Carulla.
Montserrat Serra-Batiste, first author of the paper, says, "We don't know whether this species is neurotoxic, but now that we know that it adopts a specific barrel-like structure, we can study it in the context of the disease since we know what to look for."
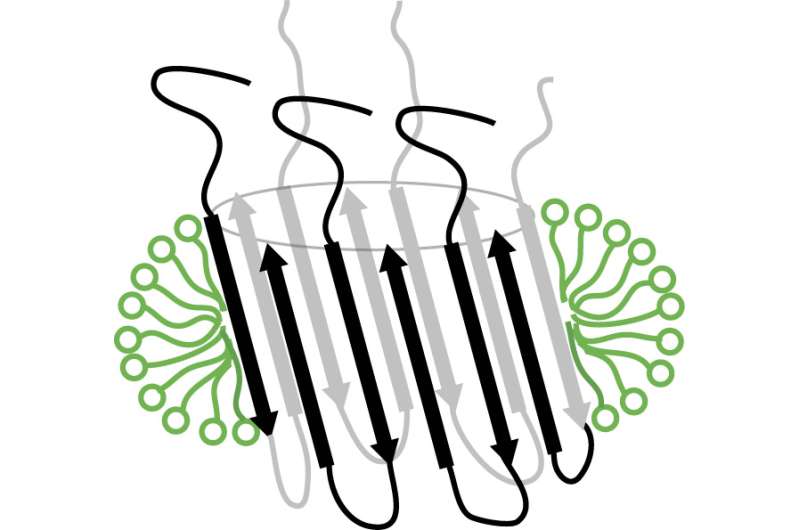
The next steps involve obtaining the 3-D structure of the aggregate and obtaining specific antibodies that recognize it. Afterwards, the researchers will study the function of this aggregate in cultured neurons derived from patients with Alzheimer's disease. These will be decisive experiments for the validation of this specific amyloid-beta aggregate as a therapeutic target.
More information: Montserrat Serra-Batiste et al. Aβ42 assembles into specific β-barrel pore-forming oligomers in membrane-mimicking environments, Proceedings of the National Academy of Sciences (2016). DOI: 10.1073/pnas.1605104113