FDA explains pros, cons of permanent birth control
(HealthDay)—Women need to carefully consider the benefits and risks of permanent birth control devices, the U.S. Food and Drug Administration says.
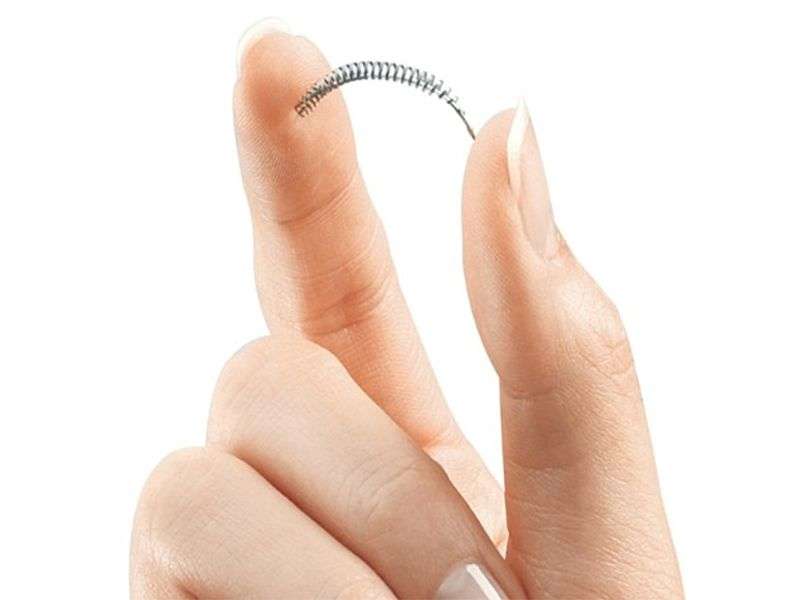
The agency recently introduced labeling changes for one such device called Essure. It consists of flexible metal coils that are implanted into the fallopian tubes, which carry eggs from the ovaries to the uterus. Within about three months, tissue forms around the coils and blocks sperm from reaching the eggs.
Because the device is made with metal, women who are sensitive or allergic to nickel or other metals should be sure to let their doctor know about their allergy, the FDA said.
The labeling changes for Essure include a boxed warning and patient decision checklist to help ensure that women receive and understand the benefits and risks of the device in order to make an informed decision about whether to use it.
An important point is that Essure is not immediately effective in preventing pregnancy. Women have to use another form of birth control for at least three months after the device is implanted. After three months, women must have an X-ray to verify the device is placed correctly and blocking the fallopian tubes, the FDA said.
Typically, Essure implantation is done in a doctor's office. The procedure doesn't require an incision and can be done without general anesthesia.
There have been reports of serious complications, the FDA said, including: poking through the fallopian tubes or uterus; persistent pain after the procedure (including pain for weeks or months after the procedure); change in menstrual cycles; symptoms similar to those of allergic reactions; and symptoms similar to those in autoimmune diseases, such as joint pain and fatigue.
Some women with complications have had surgery to remove the device, the agency reported.
Another permanent birth control option is tubal ligation—having your fallopian "tubes tied."
There are also long-acting reversible types of birth control such as the intrauterine device (IUD) and the birth control implant. Both last for several years or more, and are easy to use. If you want to become pregnant or want to stop using them, you can have the devices removed, according to the FDA.
Other types of birth control that women can consider include oral contraceptives, hormonal patches, vaginal rings, condoms and diaphragms.
"Whatever your choice in contraception, make sure you understand the risks and benefits of your options and discuss them with your health care provider," an FDA news release advised.
More information: The U.S. Office on Women's Health has more on birth control.
Copyright © 2016 HealthDay. All rights reserved.