Stopping a cancer trial early: is it for the benefit of patients or industry?
New research has identified a growing trend for trials of new cancer treatments to be stopped prematurely before the therapies’ risks and benefits have been properly evaluated.
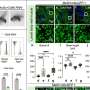
In a study, published online today (Wednesday 9 April) in the cancer journal, Annals of Oncology, Italian researchers analysed 25 randomised controlled clinical trials that had been stopped early because they had started to show a benefit to patients and found that the numbers had increased dramatically in recent years. They warn that this could lead to a systematic over-statement of the effects of treatment, and that patients could be harmed by new therapies being rushed prematurely into the clinic.
Out of 14 trials stopped because they started to show benefit to patients and published between 2005-2007, the researchers found that 11 (79%) were used to support an application for marketing authorisation at the European Medicines Agency (EMEA) and the United States Food and Drug Administration (FDA).
“This suggests a commercial component in stopping trials prematurely. In fact, this strategy (i.e. stopping trials early for benefit) could guarantee quicker access to the market for companies. On the other hand, a quicker clinical drug development may lead to an ‘immature’ benefit/risk balance of new drugs,” Dr Giovanni Apolone, one of the authors, told a news briefing yesterday (Tuesday).
Dr Apolone, head of the Laboratory of Translational and Outcome Research in Oncology, Mario Negri Institute for Pharmacological Research, Milan, Italy, continued: “When we analysed 25 trials over a ten year period between 1997 and 2007, we found a consistent increase in prematurely stopped trials – more than 50% were stopped within the last three years.
“We are aware that trials stopped early because they are showing benefit may result in the identification of promising new treatments for patients. However, findings obtained following this strategy should be considered to be preliminary results that require subsequent confirmation. We believe that only untruncated trials can provide the full level of evidence required to safely translate treatments into clinical practice. Without such evidence, unsafe and ineffective drugs could be marketed and prescribed, and patients’ health could be jeopardised.”
He pointed out that it can take several years for the long-term benefits or adverse side-effects of a treatment to become apparent, but the average study duration was 30 months among the 25 trials they analysed (with a range of between 12 and 64 months).
In their paper the authors write: “If a trial is evaluating the long-term efficacy of a treatment of conditions such as cancer, short-term benefits, no matter how significant statistically, may not justify early stopping. Data on disease recurrence and progression, drug resistance, metastasis, or adverse events, all factors that weight heavily in the benefit/risk balance, could easily be missed.
“An early stop may reduce the likelihood of detecting a difference in overall survival (the only relevant end point in this setting) because of the small sample, the possibility of crossing-over the experimental drugs, and contamination with other treatments.”
The authors said they were particularly concerned that, by the time they were stopped, five studies had enrolled less than 40% of the total number of patients planned for final analysis. “It is obvious that the risk of overestimating treatment effects increases markedly when the sample is small,” they write.
Dr Apolone said: “We believe that, in general and unless harm is being shown, cancer trials should be completed so that the correct number of patients over the correct period of time required for a significant final analysis can be recruited. Our study describes a growing phenomenon of trials stopped prematurely, which is now the subject of debate within the international scientific community.”
Professor David Kerr, the editor in chief of Annals of Oncology, told the news briefing: “We, as scientists, put a great deal of work and effort into designing appropriate clinical trials, and in all but the rarest of cases we should not rush to abandon those designs in the face of early signs of benefit. Moreover, as researchers we make an ethical commitment to patients entering studies to do the research as designed and presented to those patients. Everyone wants new and powerful therapies available quickly in clinic, but we all, patients perhaps most of all, recognise the need for good science and strong ethics.”
Professor Stuart Pocock, Professor of Medical Statistics at the London School of Hygiene and Tropical Medicine (UK), who was not involved in the study, told the news briefing: “Clinical trials need to stop early for superior benefit whenever there’s proof beyond reasonable doubt that the new treatment really is superior. That would be an ethical obligation. However, too many trials are stopped early claiming efficacy without such strong evidence being available.”
Source: European Society for Medical Oncology