July 23, 2024 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Front-line drug for ulcerative colitis found to have additional mechanism of action that until now remained elusive
Monoclonal antibodies have become indispensable in medicine to combat cancers, infectious diseases and autoimmune disorders. But the mechanism of action of a major monoclonal antibody developed for ulcerative colitis has remained elusive—until now.
The antibody is known as vedolizumab (VDZ) and it is prescribed under the brand name Entyvio, which was approved by the U.S. Food and Drug Administration a decade ago on May 20, 2014. The medication received a green light from the European Commission seven days later, on May 27, and in Japan, on May 28. It is a product of Takeda Pharmaceuticals.
Although VDZ is a front-line drug and has been successfully prescribed around the world, its broader mechanism of action has remained unclear. The drug works by binding to the cellular site that it was designed to target, but immunologists suspected something additional explained the drug's robust activity. Exactly what that was would require a full-blown study involving patients, doctors, and an interdisciplinary team of scientists.
In an article published in Science Immunology, Dr. Pablo Canales-Herrerias and a team of immunologists at the Precision Immunology Institute, a division of the Icahn School of Medicine at Mount Sinai Hospital in New York City, noted that it's critical to have a deep and more complete understanding of how the drug works.
Going into the research, Canales-Herrerias and colleagues knew that VDZ zeroes in on the α4β7 integrin, a transmembrane adhesion molecule. They also knew that when the drug interacts with its target, ulcerative colitis is controlled. To find out more about VDZ's activity, immunologists at Mount Sinai's Precision Immunology Institute were joined by a global team of scientists who aided the research and helped reveal a more complete picture of VDZ's underlying mechanism of action.
"Although VDZ is a frontline drug in the management of ulcerative colitis, our understanding of its mechanism of action remained imprecise," Canales-Herrerias wrote in Science Immunology. "The leading hypothesis that VDZ inhibits the migration of pro-inflammatory T cells to intestinal effector sites has not been demonstrated."
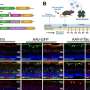
To reveal the precise molecular activities underlying successful monoclonal-antibody drug activity, Canales-Herrerias and colleagues analyzed intestinal biopsies and peripheral blood from patients with ulcerative colitis treated with VDZ.
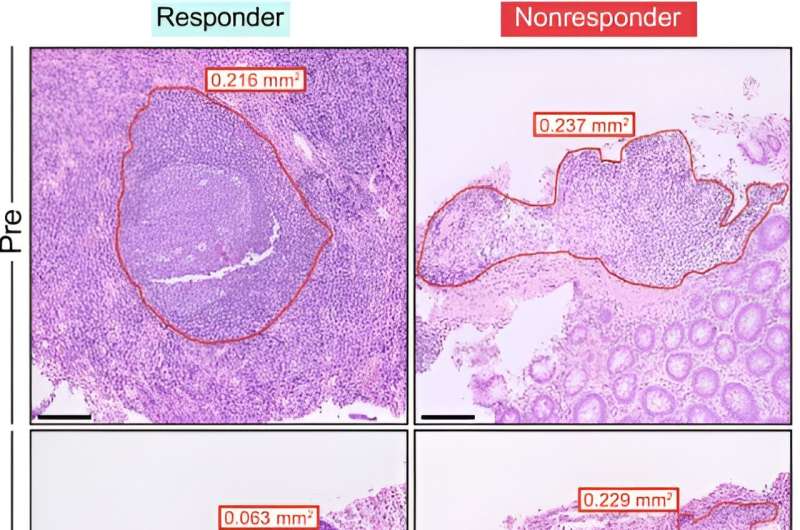
Interestingly, and surprisingly, they found that VDZ also targets gut-associated lymphoid tissue, or GALT. They learned this through a critical clue: VDZ-treated patients had fewer naïve B and T cells in intestinal tissues and decreased circulating β7+ gut-homing plasmablasts.
The team essentially discovered that VDZ targets GALT to achieve its therapeutic effect. VDZ shrinks GALT and prevents it from generating inflammatory cells, especially IgG+ plasma cells. The results suggest that targeting an immune cell-inducing site such as GALT is important in treating inflammatory bowel disease.
Ulcerative colitis is a form of inflammatory bowel disease, IBD; Crohn's disease is another. Between the two, ulcerative colitis is the most common form of IBD. It is a chronic disorder of the colon—the large intestine—marked by inflammation and ulcers. Symptoms include diarrhea, bloody bowel movements, severe cramping and weight loss. The disease affects the colon's inner lining and can range from mild to severe.
Often simply called UC, the condition tends to wax and wane in episodes of flare-ups and remissions. Patients with UC are at elevated risk for colon cancer. In the United States alone, it is estimated by the National Institutes of Health in Bethesda, Maryland that as many as 900,000 people nationwide may have the condition. An August, 2023 report in the medical journal, The Lancet noted 5 million cases globally, and the incidence is increasing worldwide, according to the article.
All of the patients participating in the Mount Sinai study were in New York City and were enrolled from the IBD Center, the Gastroenterology Department and the Digestive Endoscopy unit at Mount Sinai Hospital, in accordance with ethical guidelines. The study protocol was approved by the Mount Sinai Institutional Review Board, according to data in the study.
During the course of their research, Caneles-Herrerias and colleagues found that circulating blood and intestinal biopsy tissue taken from ulcerative colitis patients treated with VDZ, revealed a decrease in naïve B and T cells in the colon. The team confirmed the finding in a mouse model treated with anti-α4β7antibody, which limited movement of B and T cells into GALT.
Patients who responded to the drug showed signs of GALT attrition and fewer circulating and intestinal IgG+ plasma cells, which contribute to gut inflammation through the Fc?R-dependent signaling pathway.
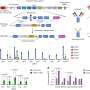
"In this study, we aimed to characterize the impact of anti-α4β7 therapy on the mucosal and circulating immune system of patients with UC and to identify correlates of therapeutic response," Canales-Herrerias said. "We profiled immune cell changes in five distinct cohorts of patients with UC."
The team concluded that GALT targeting represents a previously unappreciated mechanism of action of α4β7-targeted therapies, with major implications for this therapeutic paradigm in the future.
More information: Pablo Canales-Herrerias et al, Gut-associated lymphoid tissue attrition associates with response to anti-α4β7 therapy in ulcerative colitis, Science Immunology (2024). DOI: 10.1126/sciimmunol.adg7549
© 2024 Science X Network