This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New research offers insight on preventing brain damage in preterm babies
Mark Petersen, MD, has seen firsthand the devastating effects of brain bleeds in premature babies. It's an exceedingly common condition that affects up to 20% of infants born before 28 weeks of gestation, bringing an increased risk for developmental delays and autism.
"As a neonatologist and neuroscientist, it's frustrating that we don't have any treatments to counteract the harmful effects of bleeding in the developing brain, even though we know it often leads to lasting problems," says Petersen, director of the Neuro-Intensive Care Nursery at UC San Francisco (UCSF), associate professor of pediatrics at UCSF, and a visiting scientist at Gladstone Institutes. "Adding to this frustration, we've had very little understanding—until now—of why and how this bleeding is so closely tied to the long-term neurological issues these babies often face."
In a study appearing in Proceedings of the National Academy of Sciences, Petersen and an interdisciplinary team of physicians and scientists from Gladstone and UCSF shed light on this vexing medical condition, showing for the first time that a blood protein called fibrin blocks an essential biological process that drives brain development in early life.
When babies are born extremely prematurely, the tiny and fragile blood vessels in their brain can break, causing a hemorrhage; the younger the baby, the higher the risk. Why this happens is not fully known, but poor neurological outcomes from brain bleeding are well-established, explains Petersen, who leads his research laboratory at the UCSF Newborn Brain Research Institute.
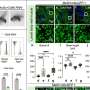
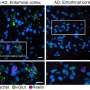
In the new study, scientists demonstrated in mouse models that fibrin, which normally works to enable blood clotting, interferes with a cell-signaling pathway that plays a vital role in neuron creation—particularly in the cerebellum. They also confirmed their findings using brain scans from nearly 60 preterm infants. No other blood protein had the same inhibitory effect on this pathway, Petersen says, which allowed the team to pinpoint fibrin as a clear therapeutic target for treating babies who experience brain bleeds.
A druggable target
The study's findings hold great significance for neonatal care, but also expand into other realms of medicine, as the signaling pathway (known as the "sonic hedgehog" pathway, or SHH) at the center of the study plays other important roles in human development and is implicated in a wide range of diseases.
At Gladstone, Petersen worked closely with the lab of Senior Investigator Katerina Akassoglou, Ph.D., who has long investigated how blood that leaks into the brain triggers neurologic diseases, essentially by hijacking the brain's immune system and setting off a cascade of harmful, often-irreversible effects leading to problems with cognition and motor functions.
"Toxic effects of blood in the developing brain point to potential new causes for neurodevelopmental disorders associated with preterm births," says Akassoglou, who also serves as director of the Gladstone-UCSF Center for Neurovascular Brain Immunology. "Neutralizing the toxic effects of fibrin in the brain presents a promising therapeutic approach for many neurological diseases—and now, we believe, may also be an approach for treating the youngest patients of all."
Akassoglou's lab previously developed a drug, a therapeutic monoclonal antibody, that acts only on fibrin's harmful properties without affecting its beneficial role in blood clotting. This fibrin-targeting immunotherapy confers protection against multiple sclerosis and Alzheimer's disease in mice. A humanized version of this first-in-class fibrin immunotherapy is already in Phase 1 safety clinical trials by Therini Bio.
"We're encouraged that we've been able to confirm the cause of lasting neurological issues in preterm babies with brain bleeding, and that we have a druggable target to potentially address this unmet need," Petersen says. "Our new study establishes the groundwork to test our therapeutic approach in developmental brain injury and other disease models with neurovascular disruption or abnormal SHH signaling."
Changing life's trajectory
Petersen has been leading the study for the past three years, starting with observations that premature babies often have a smaller cerebellum over time. By working with MRI experts and neurologists at UCSF to follow a cohort of 59 preterm infants, the team was able to show that the smaller cerebellum was linked to brain bleeding rather than infections or other possible causes.
"During this stage of early development, the cerebellum is going through a rapid change and is very sensitive to injury," Petersen says. "Anything that blocks the SHH pathway would have major implications on the brain, and it led us to investigate whether fibrin could be at play."
In addition to the MRI analyses—led by UCSF pediatric neurologist Dawn Gano, MD—the team conducted experiments in experimental models to fully understand how fibrin impacts the SHH pathway and neuron growth.
Lennart Mucke, MD, director of the Gladstone Institute of Neurological Disease, noted that the research addresses a fundamental biomedical question and has the potential to prevent persistent neurological problems stemming from a person's earliest days of life.
"The team made a really interesting discovery that may one day change the whole life trajectory of young patients with brain bleeds," Mucke says. "The focus now is on bringing this work to the clinic as quickly as possible."
More information: Olivia Weaver et al, Fibrinogen inhibits sonic hedgehog signaling and impairs neonatal cerebellar development after blood–brain barrier disruption, Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2323050121