Liver damage in hepatitis C patients could be treated with warfarin, says study
The drug warfarin may help prevent liver failure in thousands of people with Hepatitis C, according to new research.
In a study published tomorrow (1 August) in the Journal of Thrombosis and Haemostasis, researchers show that warfarin reduces the scarring on the liver caused by Hepatitis C. This scarring, or fibrosis, replaces normal liver cells and can lead to cirrhosis of the liver and ultimately liver failure.
Following the new findings in mouse models, the Imperial College London researchers are now embarking on a clinical trial of warfarin as a treatment for people with Hepatitis C, funded by the Medical Research Council (MRC).
There are an estimated 300,000 people in the UK with chronic Hepatitis C. The disease progresses much more quickly in some patients than in others and around one in five of those infected will develop cirrhosis.
Treatment to clear the infection is currently effective in only around 50 percent of patients and can have considerable unpleasant side effects such as fatigue, nausea and depression. If this treatment fails, there are no currently effective therapies to slow the progression of fibrosis.
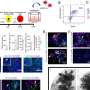
The new research looks at how warfarin affects the progression of fibrosis in mice with chronic liver injury. Warfarin is already used to prevent and treat blood clots in people with artificial heart valves, deep vein thrombosis, and a host of other conditions.
A previous study by the same researchers demonstrated that in Hepatitis C, scarring of the liver accelerates in those patients who are prone to form blood clots. This led the researchers to believe that warfarin's anti-clotting properties might enable the drug to fight the disease.
The new study showed that treatment with warfarin significantly reduces the progression of fibrosis in normal mice with chronic liver injury. It also shows that warfarin reduces the progression of fibrosis in mice with chronic liver injury and a genetic mutation known as Factor V Leiden (FVL), which causes fibrosis to progress at a much faster rate than usual because it amplifies the body's clotting mechanisms.
Professor Mark Thursz, one of the authors of the study from the Division of Medicine at Imperial College London, said: "At the moment there are a great many people with Hepatitis C who have no treatment options left and it would transform their lives if we could prevent them from developing liver failure. We are looking forward to seeing the results of our upcoming trial in humans now that we've had such promising results in the trial in mice."
Dr Quentin Anstee, an MRC Clinical Research Fellow and the corresponding author of the study from Imperial College London, added: "If we have positive results from the new trial, we will have a potential treatment that is already available and very cheap, and which should be safe enough for people to take. If we are successful in Hepatitis C patients, we are hopeful that such treatment might benefit people with liver damage from other causes, and this is something we would be keen to study further."
The researchers are recruiting 90 patients for the new trial who have undergone a liver transplant as a result of liver failure caused by hepatitis C. A third of such patients progress very rapidly to fibrosis following transplantation.
The researchers hope that treating these patients with warfarin will prevent this liver damage and improve their prognosis. Transplant patients have a liver biopsy every year following transplantation to assess their progress, and the researchers will analyse data from this biopsy to establish the effectiveness of the warfarin treatment. The two-year trial will take place across five centres including Imperial College Healthcare NHS Trust, which has integrated with Imperial College London to form the UK's first Academic Health Science Centre.
The trial is taking place in transplant patients because the researchers estimate that it would take 10-15 years to conduct a trial in patients in whom the disease was progressing at a normal rate.
Source: Imperial College London