Scientists deliver toxic genes to effectively kill pancreatic cancer cells
A research team, led by investigators at the Department of Surgery at Jefferson Medical College of Thomas Jefferson University and the Kimmel Cancer Center at Jefferson, has achieved a substantial "kill" of pancreatic cancer cells by using nanoparticles to successfully deliver a deadly diphtheria toxin gene. The findings – set to be published in the October issue of Cancer Biology & Therapy – reflect the first time this unique strategy has been tested in pancreatic cancer cells, and the success seen offers promise for future pre-clinical animal studies, and possibly, a new clinical approach.
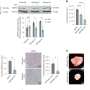
The researchers found that delivery of a diphtheria toxin gene inhibited a basic function of pancreatic tumor cells by over 95 percent, resulting in significant cell death of pancreatic cancer cells six days after a single treatment. They also demonstrated that the treatment targets only pancreatic cancer cells and leaves normal cells alone, thus providing a potential 'therapeutic window.' Further, they are targeting a molecule that is found in over three-quarters of pancreatic cancer patients.
"For the pancreatic cancer world, this is very exciting," says the study's lead author, molecular biologist Jonathan Brody, Ph.D., assistant professor, Department of Surgery at Jefferson Medical College of Thomas Jefferson University, who works closely with the Samuel D. Gross Professor and Surgeon, Charles J. Yeo, M.D. "There are no effective targeted treatments for pancreatic cancer, aside from surgery for which only a minority of patients qualify. We are in great need of translating the plethora of molecular information we know about this disease to novel therapeutic ideas."
Pancreatic cancer is the fourth leading cause of cancer-related mortality in the U.S., reflecting the generally short survival time of patients - often less than a year from diagnosis.
This approach was originally developed in ovarian cancer cells by study co-author Janet Sawicki, Ph.D., a member of the Kimmel Cancer Center, and professor at the Lankenau Institute for Medical Research in Wynnewood, Pennsylvania. She and her group had recent success in reducing the size of ovarian tumors following treatment with diphtheria toxin nanoparticles.
The strategy is based on the fact that both ovarian and pancreatic cancer cells significantly over-express a protein found on the cell membrane, called mesothelin. The function of that molecule is unknown, but it is found in the majority of pancreatic tumors and ovarian cancer tumors. Other solid tumors also express mesothelin, but not at such a high rate.
"We don't know completely why cancer cells repeatedly turn on mesothelin genes to produce these membrane proteins, but it gives us a way to fool the cell and hijack its machinery, to trick it into making other more potent genes that will be detrimental to the cancer cells," Brody says.
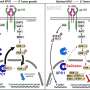
To do that, the researchers devised an agent that consists of a bit of mesothelin DNA connected to the gene that produces the toxin from diphtheria, a highly contagious and potentially deadly bacteria, which is now controlled through childhood DPT vaccination. "Naked" DNA is then coated in a polymer to form nanoparticles that are taken up by the cancer cells.
Inside the cells, the agent performs its trickery. The nanoparticles biodegrade and the cell machinery senses genetic material from mesothelin. It activates the diphtheria toxin gene, which then turns on production of the toxin which allows the toxin to then do its work on the cancer cells, Brody says. Within 24 hours of delivery, the toxin disrupted production of protein machinery by over 95 percent, and within six days, a number of cancer cells die or are arrested.
"The cancer thinks it is turning on mesothelin and once it gets started reading that genetic code, it can't stop," he says. "So it will read the bacteria's DNA and produce the toxin which shuts down protein production in the cancer cells."
"It worked well in our cell culture models and now we are moving into pre-clinical experiments," Brody says.
The agent will not attack normal cells because the molecular machinery needed to turn on mesothelin is not found in normal cells, Brody says. Additionally, Sawicki has modified the diphtheria DNA to ensure that toxin that might be released from dying cancer cells is not taken up by healthy, normal cells.
But the researchers are now perfecting even more stringent measures to ensure safety, he says. "We can't help being hopeful," he says. "Our findings suggest that such a strategy will work in the clinical setting against the majority of pancreatic tumors."
Source: Thomas Jefferson University