Studies show novel device may enhance chemotherapy treatment in brain tumors
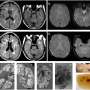
NovoCure Ltd. presented results yesterday evaluating the Novo-TTF device in vitro and in a pilot clinical trial that showed the device enhanced the efficacy of standard chemotherapy (temozolomide) treatment in newly-diagnosed glioblastoma multiforme (GBM) patients. When used in combination with standard chemotherapy, the Novo-TTF, a non-invasive medical device that uses low intensity alternating electric fields to destroy cancer cells, enhanced the anti-tumor effects of standard chemotherapy, thus prolonging time to disease progression and extending survival.
This pilot trial, which was presented during the Society of Neuro-Oncology (SNO) annual meeting yesterday in Las Vegas, NV, showed that combination therapy prolonged time to disease progression by nearly 31 months and increased survival by more than 25 months compared to historical results of patients receiving standard chemotherapy alone. Patients in the study did not experience any device-related, systemic, adverse events. The in vitro study, which was also presented during the annual meeting, mirrored these effects when applying both treatments together to GBM cells in culture.
"The pilot study further demonstrated that the NovoTTF device can be applied over an extended period of time without significant toxicity. These results are promising as NovoCure continues to evaluate the use of the device in combination with standard-of-care chemotherapies," said Eilon Kirson, MD, Medical Director, NovoCure Ltd. "This data adds to the growing body of evidence of Novo-TTF's potential to enhance standard therapy and potentially improve patient outcomes."
The Novo-TTF device disrupts cancer cell proliferation and tumor growth by generating low intensity, intermediate frequency, alternating electric fields within a tumor. These electric fields exert forces on polar structures within the dividing cancer cells that prevent tumor growth. In pre-clinical and clinical studies to date, the electric fields have shown no effect on non-dividing, healthy cells in the same region, suggesting that the device can treat cancer without harming surrounding tissue, unlike chemotherapies that are typically associated with high toxicity resulting in serious side effects. The Novo-TTF is currently an investigational medical device and is not yet FDA approved.
NovoCure recently published data from its human pilot study for patients with later stage GBM tumors that recurred after surgery and radiation. The results of this study preliminarily indicate that the Novo-TTF more than doubled the median overall survival rates for recurrent GBM patients relative to historical data.
NovoCure is currently conducting a Phase III clinical trial at more than 20 centers in the US and Europe for patients with recurrent GBM and is planning to launch a Phase III clinical trial to evaluate Novo-TTF treatment in combination with standard chemotherapy in patients with newly diagnosed GBM in 2009. Please refer to www.novocuretrial.com or call 1 (800) 978-0265 for more information on the ongoing trial.
SNO STUDY DETAILS
The in vitro study looked at the effects of TTFields alone and in combination with DTIC, a precursor of MTIC (the active metabolite of Temozolomide) in human GBM cells. The results demonstrated that, when applied together with DTIC, TTFields drastically increased the sensitivity of GBM cells in culture to DTIC.
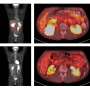
The open-label, single-arm prospective pilot clinical trial evaluated the efficacy and safety of the Novo-TTF device in combination with standard chemotherapy (temozolomide) in 10 patients with newly diagnosed GBM. The results of the study were compared to concurrent and historical control outcomes of standard chemotherapy when given alone. All patients were followed on a monthly basis and received treatment with the device for up to 18 months. The primary endpoints were feasibility, toxicity, time to disease progression and overall survival. No device-related, systemic, adverse events were noted throughout treatment with the Novo-TTF device. A mild to moderate skin irritation appeared beneath the device electrodes in all patients.
Median time to disease progression was 155 weeks in patients treated with combined standard chemotherapy and the Novo-TTF device, compared to 31 weeks in concurrent control patients treated with standard chemotherapy alone. Half of patients were still progression-free at the end of the trial. Median overall survival was greater than 40 months in the combination trial, compared to 14.7 months reported for historical controls. Eight of the 10 patients were still alive at the end of this study.
Source: Edelman Public Relations