This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A new 'one-two punch' method for improving checkpoint inhibitor therapy for cancers, including Hodgkin lymphoma
Checkpoint inhibitor therapies can be thought of as the molecular "brake release" for the immune system. These drugs eliminate the protein barriers that impede the immune system from recognizing and targeting cancer cells in the body. While there are multiple checkpoint inhibitors approved to treat different types of cancer, many patients don't respond or develop resistance to available regiments.
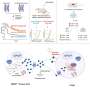
A Scripps Research team has now found that ruxolitinib, an approved immunosuppressive drug, supercharged T-cell responses when used alongside checkpoint inhibitors—boosting their effectiveness in fighting cancer. These findings, published in Science, are supported by a Phase I clinical trial of patients with Hodgkin lymphoma, as well as preclinical models. The paper is titled "JAK inhibition enhances checkpoint blockade immunotherapy in patients with Hodgkin lymphoma."
"There's a lot of activity in developing the next generation of immunotherapies, and we're looking beyond therapeutics that target T cells directly," says co-senior author John Teijaro, Ph.D., a professor in the Department of Immunology and Microbiology at Scripps Research.
T cells are produced by the immune system to fight off infections, as well as cancer. Patients often stop responding to checkpoint immunotherapy when their T cells begin to wane.
This phenomenon, called T-cell exhaustion, happens as T cells become chronically exposed to cancer cells. But based on the results of previous work, Teijaro and his research team wondered whether a JAK inhibitor—like ruxolitinib—could increase T-cell production, while also improving checkpoint inhibitors and their "brake release" effects.
JAK enzymes are important parts of the JAK/signal transducer and activator of transcription (STAT) pathway—a chain of interactions between cells and proteins that are essential for immune cell development. Dysregulation of the pathway is associated with both inflammation and cancer. JAK inhibitors restrict signals believed to cause inflammation, resulting in the immune system "calming down."
"A lot of this started about 11 years ago, when we originally found that blocking a cytokine that signals through the JAK/STAT pathway, type 1 interferon, can promote immune responses and hasten virus clearance," says Teijaro. Although JAK inhibitors are typically used to treat inflammatory diseases, there's a known genetic link between JAK mutations and cancer, he adds.
To determine which existing JAK inhibitors could restore the function of exhausted T cells, Teijaro and his team turned to ReFRAME, a drug repurposing library built by Calibr-Skaggs, the drug discovery and development arm of Scripps Research. ReFRAME permits researchers to rapidly sort through thousands of existing FDA-approved drugs and determine if they could treat any other major illnesses. Using ReFRAME, the researchers identified ruxolitinib as a contender.
Through a range of preclinical models in which mice had various forms of cancer and persistent viral infections, the researchers found that compared with checkpoint therapy alone, combining the treatment with ruxolitinib increased both the number of T cells and natural killer (NK) cells—another type of immune cell that limits the spread of cancer.
With this preclinical data in hand, the team partnered with Veronika Bachanova, MD, Ph.D., at the University of Minnesota who had initiated a Phase I clinical trial of 19 patients with Hodgkin lymphoma who failed to respond to checkpoint inhibitors or relapsed following an initial response.
"Among patients with all types of cancer, fewer than 20% respond to checkpoint inhibitors. Even in cancer types that typically respond well, such as Hodgkin lymphoma, about 10% to 20% of patients don't respond to checkpoint inhibitors, and they have to be treated with a chemotherapy that is fairly nonspecific and is not curative," explains first author Jaroslav Zak, a postdoctoral fellow at Scripps Research. "It's very difficult to treat these patients."
But two years after starting a treatment regimen that combined ruxolitinib with the checkpoint inhibitor nivolumab—a current standard of care—87% of patients were still alive, and 46% stopped exhibiting signs of cancer progression altogether.
"Anecdotally, we know for sure that at least one patient had a very good response that lasted beyond the two years of the clinical trial," says Zak. "Unlike chemotherapy, this treatment didn't just slow down the disease but actually reversed it."
Myeloid cells, a type of immune cell from the bone marrow, are among the body's most important lines of defense against infection. But cancer cells often hijack myeloid cells, which leads to tumor growth and metastasis.
A high number of myeloid suppressor cells—which are found in many types of tumors and cause weak responses to immune checkpoint inhibitors—as well as a high ratio of neutrophil-to-lymphocyte cells are tied to poor prognosis of several cancers, including Hodgkin lymphoma. But using the ruxolitinib combination therapy resulted in a reduction of both indicators, while also promoting functional T cells.
"We're now enlisting myeloid cells as helpers for immunotherapy, as it seems that in order for T cells to increase in number and functionality, ruxolitinib needs to modulate the myeloid cells," explains Teijaro.
These findings, however, were unexpected. For one, past research showed that ruxolitinib didn't work on its own to treat cancer.
"Ruxolitinib is actually an immunosuppressive drug that's clinically approved for chronic graft-versus-host disease, so the fact that we saw immune-enhancing effects in patients treated with this drug using combination therapy was definitely surprising," continues Zak.
"This suggests that some drugs can actually have immune-enhancing effects, even if their primary indication is to relieve inflammatory disease pathology."
Building on their success, the researchers plan to examine whether other JAK inhibitors are even more effective than ruxolitinib at treating cancer. They're also designing clinical trials to test the efficacy of ruxolitinib combined with checkpoint inhibitors in other forms of cancer, including those with solid tumors.
"It's very rare to have supporting evidence with preclinical data and a clinical trial in one paper," says Teijaro. "I've been doing this for decades, and I've never had that happen in my career. Our results are particularly exciting because we are already seeing patients benefit from the combination and we believe this could be applied to several immunotherapy resistant cancers."
More information: Jaroslav Zak et al, JAK inhibition enhances checkpoint blockade immunotherapy in patients with Hodgkin lymphoma, Science (2024). DOI: 10.1126/science.ade8520. www.science.org/doi/10.1126/science.ade8520