This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Team presents promising results from first clinical trial of next-gen antibody in patients with advanced solid tumors
Initial data from the first phase 1 trial of the bispecific antibody FS222 demonstrate that it is a drug with a manageable safety profile and promising antitumour activity, especially in patients with metastatic cutaneous melanoma refractory to immunotherapy with anti-PD1 immune checkpoint inhibitors.
Promising antitumor activity data, especially in melanoma patients
The ongoing trial has already included 104 patients with various types of tumors who had received between one and seven previous courses of treatment. Preliminary results from this phase 1 trial indicate partial or complete objective response rates in patients with melanoma, non-small cell lung cancer, ovarian cancer, triple-negative breast cancer, liposarcoma, and colon cancer.
The overall response rate for all tumor types was 17%. "However," adds Dr. Elena Garralda, director of the Molecular Cancer Therapy Research Unit UITM-CaixaResearch, who led this study, "the results stand out in patients with advanced cutaneous melanoma who had not responded to immunotherapy with immune checkpoint inhibitors". In these patients, the overall response rate was 47.4%, and the disease was controlled in 68.4 % of the patients.
"Although these are very preliminary results, they are indeed very promising, especially in patients with melanoma who do not respond to conventional immunotherapy," comments Dr. Garralda.
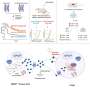
The adverse effects associated with the treatment show an acceptable and manageable safety profile for the drug. Regarding pharmacology, modulation and increase of tumor T cells were observed in biopsies of treated patients, confirming the activation of the immune response against the tumor.
Innovative design to reactivate the immune response against the tumor
"Although the advent of immunotherapy has been a revolution in the landscape of cancer treatment, to this day, most patients receiving immune checkpoint inhibitors do not respond to the treatment or relapse. Hence, the need to continue researching to find new immunotherapy strategies that offer greater benefits to a larger number of patients," explains Dr. Garralda.
FS222 is an innovative next-generation bispecific antibody. Its tetravalent structure allows it to inhibit the PD-L1 immune checkpoint on one side and presents an agonist or enhancer of the immune response on the other. This enables it to very potently and selectively activate the patient's immune system against tumor cells.
"Next steps include a better optimization of dose selection and further evaluation of the efficacy profile of FS222 in patients with melanoma and other tumor types to confirm activity in a larger number of patients," concludes Dr. Garralda, who presented these preliminary data from the first human trial of this innovative antibody at the 2024 ASCO Annual Meeting, held in Chicago from 31 May to 4 June.