This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Study: Key mechanisms of action differences in immune checkpoint inhibitor combination therapies for advanced melanoma
Checkpoint inhibitors that activate the immune system to target cancer cells for destruction have revolutionized the treatment landscape for patients with advanced melanoma, leading to more options and improved patient survival. Despite the approval of several immune checkpoint inhibitor regimens for melanoma, scientists do not completely understand their anticancer effects.
In a study published in the Journal for ImmunoTherapy of Cancer, a team of researchers from the Donald A. Adam Melanoma and Skin Cancer Center of Excellence at Moffitt Cancer Center reveals differences in the mechanisms of action of two FDA-approved immune checkpoint inhibitor combination therapies for advanced melanoma. The study is titled "Differential requirements for CD4+ T cells in the efficacy of the anti-PD-1+LAG-3 and anti-PD-1+CTLA-4 combinations in melanoma flank and brain metastasis models."
Several types of immune checkpoint inhibitors have been approved to treat advanced melanoma, including drugs that target the proteins PD-1 and PD-L1. More recently, results from clinical trials revealed that PD-1/PD-L1 inhibitors in combination with other immune checkpoint inhibitors that target the proteins CTLA-4 or LAG-3 result in better patient outcomes than PD-1/PD-L1 agents alone, leading to their approvals to treat advanced melanoma.
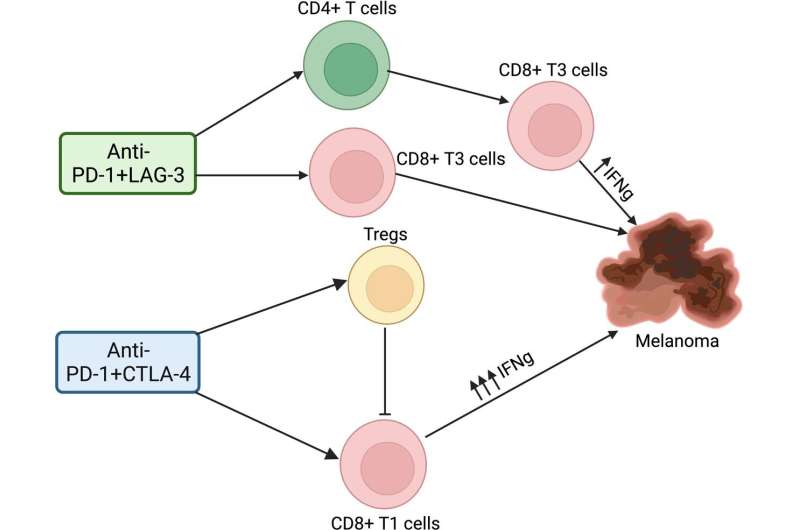
While anti-PD-1/CTLA-4 and anti-PD-1/LAG-3 therapies work in generally similar ways to stimulate the immune system, mechanistic differences are likely as CTLA-4 and LAG-3 have different cell expression patterns, binding partners and signaling activity.
The Moffitt researchers wanted to analyze the mechanisms of action of anti-PD-1/CTLA-4 and anti-PD-1/LAG-3 treatments in advanced melanoma and identify the specific subtypes of immune cells that become activated.
The immune system is composed of many different types of immune cells that work in conjunction to promote and inhibit immune responses. The primary mediators of the anticancer effects of immune checkpoint inhibitors are T cells, which include cytotoxic T cells called CD8 T cells that kill infected cells or tumor cells; helper T cells called CD4 T cells that coordinate immune responses between CD8 T cells and other immune cells; and regulatory cells called Tregs that can suppress an immune response.
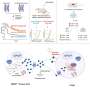
The researchers performed laboratory experiments with mouse models of melanoma and melanoma brain metastases to identify the precise immune cells that become activated during treatment. They discovered that the two immune checkpoint inhibitors combination regimens have different mechanisms of action mediated through different effects on CD4 T cells.
The anti-PD-1/LAG-3 drug combination required the presence of CD4 T cells for its anticancer effects in both cutaneous melanoma and brain metastases melanoma, while the anti-PD-1/CTLA-4 combination did not require their presence.
Furthermore, the anti-PD-1/LAG-3 regimen resulted in decreased Treg cell activity and increased CD4 helper T cell activity that led to CD8 T cell activation, while the anti-PD-1/CTLA-4 regimen resulted in the accumulation and the direct activation of more cytotoxic CD8 T cells.
While most research has focused on the importance of CD8 T-cell activity to immune checkpoint inhibitor anticancer effects, these combined observations support the growing evidence that CD4 helper T cells also play an important role in the effects of immune checkpoint inhibitors.
These data reveal the key differences that can be used to optimize outcomes for patients with melanoma, particularly among patients who develop drug resistance.
"Many patients exhibit upfront or acquired resistance to standard of care anti-PD-1 or the anti-PD-1+CTLA-4 combination, suggesting there could still be responses to a second line immune checkpoint inhibitor therapy with a different mechanism of action. These observations are of particular interest in the context of melanoma brain metastases, where additional therapeutic strategies are urgently needed," said Keiran Smalley, Ph.D., director of the Donald A. Adam Melanoma and Skin Cancer Center of Excellence at Moffitt.
More information: Manali S Phadke et al, Differential requirements for CD4+ T cells in the efficacy of the anti-PD-1+LAG-3 and anti-PD-1+CTLA-4 combinations in melanoma flank and brain metastasis models, Journal for ImmunoTherapy of Cancer (2023). DOI: 10.1136/jitc-2023-007239