HER2 levels may aid in treatment selection for metastatic breast cancer
Findings published in the December 1, 2008, issue of Clinical Cancer Research, a journal of the American Association for Cancer Research, show lapatinib benefits women with HER2-positive breast cancer, while women with HER2-negative breast cancer or those who express EGRF alone derive no incremental benefit. In addition, a misclassification of metastatic breast cancer patients by as much as 10 percent prevents some people from receiving optimal therapy.
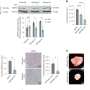
Lapatinib, an oral chemotherapy agent, inhibits both HER2 and EGRF receptors, leaving unanswered questions about which patients are more likely to benefit. Researchers at the Norris Comprehensive Cancer Center found that HER2 amplification ("HER2-positive"), but not EGRF expression, is correlated with responsiveness to lapatinib. Women with both high and low levels of HER2 amplification respond to lapatinib. However, women with HER2-negative metastatic breast cancers do not respond.
Women with HER2-postitive metastatic breast cancer who receive lapatinib and chemotherapy have shown an improvement of approximately 50 percent in progression-free survival when compared to chemotherapy alone. Unfortunately, high volume laboratories using laboratory technicians instead of pathologists to score gene amplification misclassify approximately 10 percent of HER2 amplified breast cancers as not amplified, preventing these patients from being candidates for lapatinib.
"I would like to see all women with breast cancer tested appropriately, using the best method and certified personnel, to assess the HER2 status of their breast cancer so the appropriate treatment can be selected," said Michael Press, M.D., Ph.D., Norris Comprehensive Cancer Center Harold E. Lee Chair in Cancer Research and lead author of the study.
Currently lapatinib is approved by the FDA for use only in women who have HER2-positive metastatic breast cancer who were previously treated with anthracyclines, trastuzumab and taxane.
Source: American Association for Cancer Research