Cell's split personality is a major discovery into neurological diseases
Researchers at the Université de Montreal (UdeM) and the Montreal Neurological Institute (MNI), McGill University have discovered that cells which normally support nerve cell (neuron) survival also play an active and major role in the death of neurons in the eye. The findings, published this week in The Journal of Neuroscience, may lead to more streamlined therapies for a variety of acute and chronic neurological disorders, including glaucoma and retinal artery occlusion.
In many neurodegenerative diseases, a main factor that kills neurons is excessive levels of glutamate, the most abundant excitatory neurotransmitter in many regions of the central nervous system (CNS). Diseases that occur as a result of high glutamate levels include hypoxic-ischemic brain injury (stroke), trauma, seizures, various forms of dementia and neurodegeneration. For years, the main explanation for the toxic effects of glutamate is that it overexcites neuronal cells via activation of glutamate receptors and thereby kills them.
"The most interesting aspect of our study and the reason we are so excited is that the pathway leading to glutamate-induced nerve cell death involves another vital player - namely, glial cells," says Dr. Adriana Di Polo, neuroscientist at the UdeM. "Through careful experimentation we now know that glutamate activates signaling pathways in glial cells that then lead to neuronal death."
Glial cells are the most abundant cell type in the nervous system and are traditionally thought of as 'partner' cells to nerve cells providing support, nutrients and an optimal environment. However, this study indicates that glial cells also have a more sinister side that allows them to induce or exacerbate neuronal death in pathological conditions.
"Neuronal cell death induced by glutamate is a key step in a large number of injury and disease settings and this study is important because it provides a road-map for the cellular and molecular events that allow this to occur" says Dr. Philip Barker, neuroscientist at the MNI, "The fact that specific signaling events in glial cells are important for inducing neuronal cell death is surprising and suggests new therapeutic targets for conditions that involve excitotoxicity."
The findings of the MNI and UdeM study represent a paradigm shift from the main model of excitotoxicity that has been in place for many years. Until now, the central idea has been that glutamate, which is released upon injury, binds to and activates the glutamate receptors on neurons triggering massive calcium entry and cell death. However, clinical trials targeting glutamate receptors have been disappointing suggesting that these receptors play only a minor role in triggering neuronal death.
The study, supported by the Canadian Institutes of Health Research, focused on nerve cells in the retina which convey information from the retina to the brain along the optic nerve, and are the primary link between the retina and the brain. The death of these retinal neurons from excess glutamate causes vision loss in various neurodegenerative disorders including optic neuropathies.
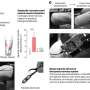
By disrupting signaling events in surrounding glial cells, the researchers were able to protect the majority of these neurons, confirming that glial cell events play a key role in death triggered by glutamate. This new understanding of the excitotoxic cascade of nerve cell death provides clear targets for successful therapeutic intervention of a wide-range of neurological and neurodegenerative diseases.
Source: McGill University (news : web)