This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New cellular mechanisms in Parkinson's disease discovered
A pair of recent studies led by Joseph Mazzulli, Ph.D., associate professor in The Ken & Ruth Davee Department of Neurology's Division of Movement Disorders, have uncovered previously unknown cellular mechanisms involved in neuronal protein aggregation and misfolding, key characteristics of Parkinson's disease.
Published in Neuron and Nature Communications, the findings highlight multiple new pathways that support pathogenesis and may serve as therapeutic targets for restoring neuronal function in patients with Parkinson's and other neurodegenerative diseases.
A hallmark of neurodegenerative diseases including Parkinson's disease and Lewy body dementia is the aggregation of a protein called alpha-synuclein, which is found in neural tissue.
In healthy cells, the protein supports neurotransmitters in traveling between neurons; however, in neurons affected by Parkinson's disease, the protein becomes misfolded and aggregates in large clumps (Lewy bodies), which can damage neurons by causing inflammation, mitochondrial and lysosomal dysfunction.
Previous work from the Mazzulli laboratory had found that alpha-synuclein-induced deficits in trafficking between the endoplasmic reticulum and Golgi leads to a buildup of proteins that are unable to exit the endoplasmic reticulum and enter the lysosome, ultimately disrupting waste disposal pathways in the cell and causing protein aggregation.
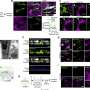
In the new study published in Neuron, Mazzulli's team aimed to identify the proteins that are susceptible to aggregation in neurons affected by Parkinson's disease by analyzing induced pluripotent stem cell (iPSC) midbrain cultures derived from patients with familial Parkinson's disease and Lewy body dementia.
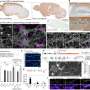
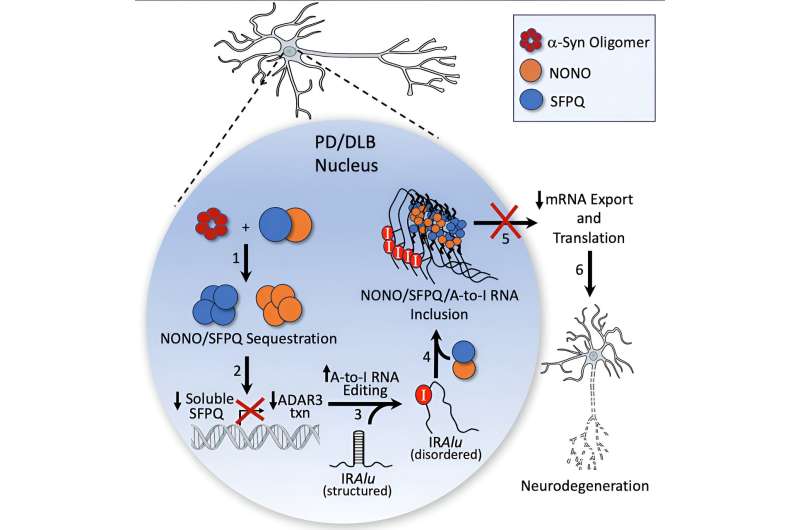
Alpha-synuclein pathology is typically found in the cytoplasm of neurons, according to Mazzulli. However, using an unbiased proteomic screening approach, his team unexpectedly discovered a set of accumulated RNA-binding proteins in the nucleus: NONO and SFPQ.
"They're interesting proteins because they bind RNA and fall within a class of flexible proteins that are made to purposely change shape between soluble forms and insoluble particles, called paraspeckles, in a reversible manner.
"In Parkinson's neurons, however, NONO and SFPQ form abnormally large insoluble inclusions in the nucleus that are unable to revert into their normal, functional soluble state," Mazzulli said.
The investigators discovered that once the proteins aggregate, they reduced the expression of the RNA editing inhibitor ADAR3 and, in turn, increased adenosine-to-inosine (A-to-I) RNA editing in the nucleus, impacting the expression and function of vital neuronal proteins.
"The field has been really focused on alpha-synuclein aggregation. We're not saying that's not important, but we're showing that there are other proteins and RNA inclusions involved in Parkinson's pathogenesis that we haven't seen before.
"Using methods like this, where we're screening patient neurons in vitro, led us to discover this novel pathology made of both RNA and protein aggregates in patient brains, as well," Mazzulli said.
"After over a quarter of century since alpha-synuclein was discovered in Lewy bodies of Parkinson's patients, our research highlights the existence of novel RNA-binding protein aggregates along with aberrant A-I edited RNA.
"This challenges our current understanding of Parkinson's pathology and provides exciting opportunities for new therapeutic targets focused on the RNA-editing pathway," said Nandkishore Belur, MS, a research associate in the Mazzulli laboratory and lead author of the study.
In the other study, published in Nature Communications, Mazzulli and colleagues aimed to better understand the mechanisms that link glucose metabolism and protein misfolding in neurons affected by Parkinson's disease.
Following a similar methodology as the previous study, Mazzulli's team analyzed neurons in iPSC midbrain cultures derived from patients with Parkinson's disease and discovered the disruption of a metabolic pathway, the hexosamine pathway, that is important for protein synthesis, transport and folding in the neuron's endoplasmic reticulum.
The hexosamine pathway produces N-linked glycans, essential molecules that support protein folding in the endoplasmic reticulum. In Parkinson's midbrain cultures, however, this N-glycosylation process was interrupted, causing protein misfolding and accumulation.
"We found a metabolic enzyme in the hexosamine pathway that was depleted, called GFPT2. It's an important enzyme and provides the precursors for N-glycosylation. If you wipe that out, protein folding in the endoplasmic reticulum is severely compromised, leading to proteome stress," Mazzulli said.
Using a pharmaceutical approach, the investigators inserted n-acetylglucosamine, a key component in the hexosamine pathway, into the neurons and found that this approach restored lysosomal activity and improved protein folding in the endoplasmic reticulum.
Going forward, Mazzulli said his team hopes to test both pharmaceutical and genetic approaches to restore expression of GFPT2 in alpha-synuclein mouse models to determine if these treatments elevate lysosomal activity and protein clearance in the brain.
"When you think about Parkinson's treatments, anything that's in the preclinical stage now or in clinical trials is targeting one pathway. Our studies show that multiple pathways are perturbed in the disease, and so we think that rescuing multiple pathways at once is essential for disease modification," Mazzulli said.
More information: Nandkishore R. Belur et al, Nuclear aggregates of NONO/SFPQ and A-to-I-edited RNA in Parkinson's disease and dementia with Lewy bodies, Neuron (2024). DOI: 10.1016/j.neuron.2024.05.003
Willayat Y. Wani et al, The hexosamine biosynthetic pathway rescues lysosomal dysfunction in Parkinson's disease patient iPSC derived midbrain neurons, Nature Communications (2024). DOI: 10.1038/s41467-024-49256-3