To regenerate muscle, cellular garbage men must become builders
For scientists at the European Molecular Biology Laboratory in Monterotondo, Italy, what seemed like a disappointing result turned out to be an important discovery. Their findings, published online this week in the journal Proceedings of the National Academy of Sciences (PNAS), provide conclusive proof that, when a muscle is injured, white blood cells called macrophages play a crucial role in its regeneration. The scientists also uncovered the genetic switch that controls this process, a finding that opens the door for new therapeutic approaches not only to sports injuries but also to diseases such as Duchenne muscular dystrophy.
Normally, macrophages - the white blood cells known for engulfing and eliminating bacteria and other infectious agents - are drawn to areas of injury. Once there, they act as garbage men, eliminating the dead cells and releasing pro-inflammatory factors, fending off infection. After clearing up the debris, macrophages stop releasing those pro-inflammatory factors, and start making anti-inflammatory factors that promote repair in the damaged area. This shift from clearing debris to promoting building is known as macrophage polarization, and Claus Nerlov, Nadia Rosenthal and colleagues proved that it is essential for muscles to regenerate properly.
“There seems to be this point of no return”, says Rosenthal: “if macrophages don’t make this switch, then the muscle won’t repair itself - you just end up with scar, instead of new tissue”.
Nerlov and his research group at EMBL were studying a protein called C/EBPb, whose production increases in response to inflammation. They had genetically engineered mice in which this boost in C/EBPb production was blocked, to see what effect this had on the development of the different cells involved in the immune system. To their dismay, the answer appeared to be ‘almost none’. The modified mice developed normally, and had normal blood cells - except their macrophages didn’t polarize. Although this result fell short of the scientists’ expectations of understanding how blood cells develop, it raised an interesting possibility in the context of Rosenthal’s research into muscle regeneration. If these mice could not repair muscle injuries properly, it would prove that macrophage polarization is indispensable for muscle regeneration.
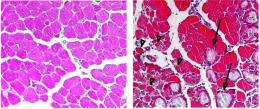
The two groups teamed up to investigate how the ability to respond to muscle injury was affected in mice whose C/EBPb production boost had been blocked. Their findings proved that macrophages still migrated to the injured site and cleared the debris, but because they failed to make that all-important switch, the muscle didn’t repair properly, becoming scarred instead.
At a stroke, the EMBL scientists confirmed the importance of macrophages in repairing muscle tissue and discovered its genetic basis. Normally, inflammatory factors trigger an increase in C/EBPb production, which in turn activates genes that cause the macrophage to polarize.
“From a medical point of view, it would seem that the trick to improve muscle repair is finding a way to increase C/EBPb production and keep it high”, Nerlov concludes, adding “if we can now figure out exactly which key genes C/EBPb controls, that will give us even more potential targets.”
As well as investigating the other steps on this molecular pathway, the scientists are currently studying a possible role for macrophage polarization in repairing heart muscle, with a view to better understanding and treating heart disease.
More information: Ruffell, D., Mourkioti, F., Gambardella, A., Kirstetter, P., Lopez, R. G., Rosenthal, N. & Nerlov, C. A CREB-C/EBP cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair, PNAS online Early Edition, 21-25 September 2009
Provided by European Molecular Biology Laboratory (news : web)