Fluorescent compounds make tumors glow
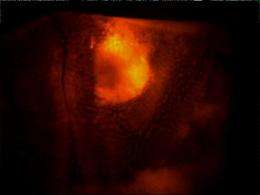
A series of novel imaging agents could light up tumors as they begin to form - before they turn deadly - and signal their transition to aggressive cancers.
The compounds - fluorescent inhibitors of the enzyme cyclooxygenase-2 (COX-2) - could have broad applications for detecting tumors earlier, monitoring a tumor's transition from pre-malignancy to more aggressive growth, and defining tumor margins during surgical removal.
"We're very excited about these new agents and are moving forward to develop them for human clinical trials," said Lawrence Marnett, Ph.D., the leader of the Vanderbilt University team that developed the compounds, which are described in the May 1 issue of Cancer Research.
COX-2 is an attractive target for molecular imaging. It's not found in most normal tissues, and then it is "turned on" in inflammatory lesions and tumors, Marnett explained.
"COX-2 is expressed at the earliest stages of pre-malignancy - in pre-malignant lesions, but not in surrounding normal tissue - and as a tumor grows and becomes increasingly malignant, COX-2 levels go up," Marnett said.
Compounds that bind selectively to COX-2 - and carry a fluorescent marker - should act as "beacons" for tumor cells and for inflammation.
Marnett and his colleagues previously demonstrated that fluorescent COX-2 inhibitors - which they have now dubbed "fluorocoxibs" - were useful probes for protein binding, but their early molecules were not appropriate for cellular or in vivo imaging.
"It was a real challenge to make a compound that is COX-2 selective (doesn't bind to the related COX-1 enzyme), has desirable fluorescence properties, and gets to the tissue in vivo," Marnett said.
To develop such compounds, Jashim Uddin, Ph.D., research assistant professor of Biochemistry, started with the "core" chemical structure of the anti-inflammatory medicines indomethacin and celecoxib. He then tethered various fluorescent parts to the core structure, ultimately synthesizing more than 200 compounds. The group tested each compound for its interaction with purified COX-2 and COX-1 proteins and then assessed promising compounds for COX-2 selectivity and fluorescence in cultured cells and in animals. Two compounds made the cut.
In studies led by senior research specialist Brenda Crews, the investigators evaluated the potential of these compounds for in vivo imaging using three different animal models: irritant-induced inflammation in the mouse foot pad; human tumors grafted into mice; and spontaneous tumors in mice.
In each case, the two fluorocoxibs - injected intravenously or into the abdominal cavity - accumulated in the inflamed or tumor tissue, giving it a fluorescent "glow."
To move the agents toward human clinical trials, the team will conduct additional toxicology and pharmacology testing and develop the tools for particular settings that are amenable to fluorescence imaging, such as skin or sites accessible by endoscope (e.g., esophagus and colon).
In the esophagus, for example, a pre-malignant lesion called Barrett's esophagus can transition to a low-grade dysplasia, then to a high-grade dysplasia, and finally to malignant cancer, which has a one-year survival of only 10 percent. For a patient with Barrett's esophagus, detecting the transition to dysplasia is critical. The problem is that dysplasia is not visibly different from the pre-malignant Barrett's lesion, so physicians collect random biopsy samples - which might miss areas of dysplasia.
"If instead, the physician could look through the endoscope and see a nest of cells lighting up with these fluorocoxibs - that is where they could biopsy," Marnett said.
"Because COX-2 levels increase during cancer progression in virtually all solid tumors, we think these imaging tools will have many, many different applications."
The investigators also are exploring using the compounds to target delivery of chemotherapeutic drugs directly to COX-2-expressing cells - by tethering an anti-cancer drug instead of a fluorescent marker to the COX-2 inhibitor core.














