In breakthrough, nerve connections are regenerated after spinal cord injury
Researchers for the first time have induced robust regeneration of nerve connections that control voluntary movement after spinal cord injury, showing the potential for new therapeutic approaches to paralysis and other motor function impairments.
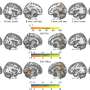
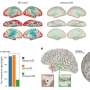
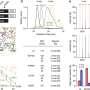
In a study on rodents, the UC Irvine, UC San Diego and Harvard University team achieved this breakthrough by turning back the developmental clock in a molecular pathway critical for the growth of corticospinal tract nerve connections.
They did this by deleting an enzyme called PTEN (a phosphatase and tensin homolog), which controls a molecular pathway called mTOR that is a key regulator of cell growth. PTEN activity is low early during development, allowing cell proliferation. PTEN then turns on when growth is completed, inhibiting mTOR and precluding any ability to regenerate.
Trying to find a way to restore early-developmental-stage cell growth in injured tissue, Zhigang He, a senior neurology researcher at Children's Hospital Boston and Harvard Medical School, first showed in a 2008 study that blocking PTEN in mice enabled the regeneration of connections from the eye to the brain after optic nerve damage.
He then partnered with Oswald Steward of UCI and Binhai Zheng of UCSD to see if the same approach could promote nerve regeneration in injured spinal cord sites. Results of their study appear online in Nature Neuroscience.
"Until now, such robust nerve regeneration has been impossible in the spinal cord," said Steward, anatomy & neurobiology professor and director of the Reeve-Irvine Research Center at UCI. "Paralysis and loss of function from spinal cord injury has been considered untreatable, but our discovery points the way toward a potential therapy to induce regeneration of nerve connections following spinal cord injury in people."
According to Christopher & Dana Reeve Foundation data, about 2 percent of Americans have some form of paralysis resulting from spinal cord injury, which is due primarily to the interruption of connections between the brain and spinal cord.
An injury the size of a grape can lead to complete loss of function below the level of injury. For example, an injury to the neck can cause paralysis of arms and legs, loss of ability to feel below the shoulders, inability to control the bladder and bowel, loss of sexual function, and secondary health risks including susceptibility to urinary tract infections, pressure sores and blood clots due to an inability to move the legs.
"These devastating consequences occur even though the spinal cord below the level of injury is intact," Steward noted. "All these lost functions could be restored if we could find a way to regenerate the connections that were damaged."
He and his colleagues are now studying whether the PTEN-deletion treatment leads to actual restoration of motor function in mice with spinal cord injury. Further research will explore the optimal timeframe and drug-delivery system for the therapy.