Researchers identify breast cancer culprits
Scientists have discovered an accomplice in breast cancer - a master control switch with the power to set off a cascade of reactions orchestrated by a cancer-causing gene (or oncogene) named Wnt1. This executive molecule and its modus operandi are reported in back-to-back papers featured on the cover of the August 15 issue of Cancer Research.
"These papers are about the regulation of a Wnt oncogene," explains lead author Rakesh Kumar, Ph.D., professor and the Catharine Birch & William McCormick Chair of the department of biochemistry and molecular biology at The George Washington University School of Medicine and Health Sciences. Now, Kumar and his team describe how a master switch sparks a type of Wnt signaling in breast cancer. Moreover, this master control switch may help explain why increased levels of a protein called MTA1 (metastasis-associated protein 1) are oncogenic in certain types of breast cancer.
Like many molecular pathways underlying cancer, Wnt pathways govern normal processes like embryonic development and the communication between cells in healthy people. For reasons little understood, however, certain types of Wnt proteins sometimes go awry, sending off cascades of signals that turn normal cells into cancerous ones. Researchers often find evidence of Wnt pathway activation when they analyze what genes are turned on in tumors. Although Wnt has been connected with breast cancer for nearly 30 years, however, the signals (other than mutations) that trigger it remain largely unknown.
Kumar and his team have implicated MTA1 and a shorter variation of the protein, MTA1s, in Wnt1 (a type of Wnt) pathway activation. MTA1 belongs to the MTA family of genes, which help a range of cancers progress in a variety of ways. Before this study, researchers knew that MTA1 levels were higher than normal in breast, ovarian, prostate, colorectal, gastric, liver tumors, and more. But they still didn't know everything about what MTA's were doing there.
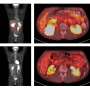
In the current studies, funded by a grant from the National Cancer Institute, Kumar's team finds that MTA1 expression triggers cancer-causing signals from Wnt1 in human breast cancer cells. This Wnt1 signaling cascade leads to tumors, they demonstrate, by showing that 8.8 percent of mice bearing artificially elevated levels of MTA1s grew tumors in their mammary glands.
To get down to the details, Kumar and his fellow researchers show that MTA1 and MTA1s activate the cancer-causing pathway by reducing the levels of a protein known as Six3. This protein is known to inhibit Wnt1 in brain cells, but in their study involving breast cancer cells, it inhibited Wnt1 in a rather non-intuitive way. Six3 normally puts the brakes on Wnt signaling, and so when MTA1 obstructed Six3, Wnt1 signals let loose. In addition, the team found that MTA1s also promoted Wnt signaling directly and through another known Wnt-related pathway - namely ERK-mediated GSK3ß.
Because inflammation may drive MTA1, and since inflammation is believed to drive certain forms of cancer, Kumar's work suggests one possible reason for why worsening cancer progression has been correlated with other inflammation-inducers. "We've raised the next level question," says Kumar, "and now we're going back into the lab to ask if this pathway plays a role in inflammation-related cancer."