Hijacked antiviral protein subverts energy production to aid viral infection
(Medical Xpress) -- Viruses are notorious for entering cells, taking over their internal machinery, and turning them into virus manufacturing centers. But new research by Howard Hughes Medical Institute investigator Peter Cresswell reveals how human cytomegalovirus takes this gambit one step further, turning a protein that host cells use to protect themselves against viruses into a saboteur that supports viral infection.
The research shows how the virus hijacks an antiviral protein and uses it to enhance infection by slowing down the host cell’s energy production. Cresswell’s team published their research in the April 28, 2011, issue of Science Express.
The name of the co-opted protein is viperin, first discovered in Cresswell’s Yale University laboratory in the late 1990s. Despite its label, the protein has nothing to do with snakes. The name stands for virus inhibitory protein, endoplasmic reticulum-associated, interferon-inducible. Its production is switched on by the immune system in response to infection. Since its discovery, Cresswell’s group and others have found that viperin can protect cells against a number of viral invaders, including influenza, herpes simplex, and, in some situations, cytomegalovirus.
Cytomegalovirus is a common virus that causes few to no symptoms in most infected people. It can cause serious disease, however, when it infects people whose immune systems are compromised or babies before they are born. Cresswell’s team became interested in cytomegalovirus when another group showed that when it infects cells, viperin, along with several other genes, is quickly switched on. "We saw this and immediately wondered why a virus was inducing expression of a gene also induced by [the immune protein] interferon," he said.
Cresswell has found that the virus has a complicated relationship with viperin. If viperin is present in the cell at the time the virus invades, the cell resists infection. But if cytomegalovirus invades and viperin is not yet being produced, the virus now runs the show.
Cresswell’s lab showed in 2001 that cytomegalovirus directly promotes viperin production. It also causes viperin to leave its home in the endoplasmic reticulum – a folded membrane sheet where proteins and lipids are manufactured – and move into the Golgi apparatus, an organelle that packages macromolecules after they have been synthesized by the cell.
The assumption has been that cytomegalovirus, which needs the host cell’s endoplasmic reticulum to start churning out viral proteins, does this to get the anti-viral protein out of its way.
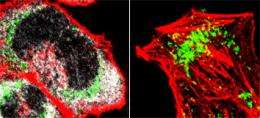
But with improved tools for tracking viperin’s movements through the cell, Jun-Young Seo, first author of the Science Express paper, discovered that viperin has an opportunity to contribute more directly to viral infection. He found that before viperin moved to the Golgi apparatus in infected cells, it made a stop in the mitochondria, the organelles in which energy is produced.
Seo knew that a cytomegalovirus protein called vMIA – for viral mitochondrial inhibitor of apoptosis – also travels to mitochondria. Previously, vMIA had been blamed for many of the effects cytomegalovirus infection has on cells, including a reduction in energy production and loss of the cell’s cytoskeleton. Seo’s findings, however, suggest vMIA’s most important role may be to transport viperin.
“He found that vMIA is essentially a bicycle and viperin hopped on and did its thing,” Cresswell explained.
Seo discovered that once viperin arrives in the mitochondria, it sabotages energy production. By altering the way the cell metabolizes fatty acids, viperin reduces the cell’s ability to generate the cellular fuel known as ATP. Because the cell needs ATP to build and maintain its structural skeleton, this ultimately causes the cytoskeleton to dissolve, creating a more hospitable environment for cytomegalovirus manufacture. Cresswell says this may be the first evidence of a virus manipulating the metabolic state of the cell for its own advantage.
To determine how critical viperin is to the virus’s ability to infect human cells, Cresswell’s lab created cells that lacked the protein. When they exposed the cells to the virus, they found that when cytomegalovirus had no viperin it could call into its service, the infection lost its vigor. It needed viperin to help it do its worst.
The new findings offer researchers a better understanding of how cytomegalovirus interacts with host cells, but have been puzzling to Cresswell and his colleagues when they consider viperin’s role in uninfected cells, he says.
“A virus doesn’t invent things. Viruses hijack things. They steal things,” Cresswell said. “So in what normal circumstance does viperin go to the mitochondria? And when it’s there, why would it induce metabolic change? Why would the cell require this metabolic change in a normal physiologic situation?
“We don’t know. It’s a black box,” Cresswell acknowledged. “We toss that around the lab all the time and we’re doing experiments to poke at the question, but we don’t have a fantastically good hypothesis.”