Nerve cells grown from stem cells give new insight into Parkinson's
Oxford University researchers have succeeded in using stem cell technology to grow nerve cells in the laboratory from initial skin samples taken from Parkinson’s patients. It’s the first large-scale effort of its kind in the UK.
The advance will allow nerve cells that are just like those of the people with Parkinson’s to be studied intensively in the laboratory in ways that weren’t possible before.
The study aims to investigate what processes are making specific nerve cells, called dopamine neurons, die off as the disease progresses.
"We can’t take bits of people’s brains when they are alive, of course," says Dr. Richard Wade-Martins of the Oxford Parkinson’s Disease Centre, who led the work. "So we never have been able to study dopamine neurons from a patient."
"We now have a platform to understand what happens in the cells in disease and identify new pathways that could lead to potential targets for new therapies."
BBC News followed the steps in the process with one patient from Oxfordshire. A skin biopsy was provided by Mr Derek Underwood, who used to work for a manufacturer of MRI scanners before his early retirement through Parkinson’s.
Skin cells from Mr. Underwood’s biopsy were then grown up in the lab. At the appropriate point, using an approach recently developed by Japanese scientists, the skin cells were ‘reprogrammed’ to revert back to a stem-cell-like state.
The great power of these induced pluripotent stem cells, or iPS cells, is that they are able to form just about any of the specialised types of cell present in the body. That allowed the Oxford team to guide the iPS cells to develop, or ‘differentiate’, into nerve cells that are essentially identical to the Mr. Underwood’s own nerve cells in his brain.
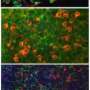
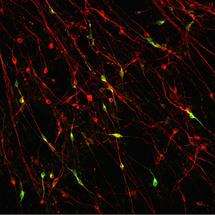
Some of the nerve cells generated are dopamine neurons, the ones involved in Parkinson’s disease.
Each of the stages in the process – skin cells to iPS cells to nerve cells – are visible under the microscope as they all look very different. The clearly defined circular colony of stem cells gradually breaks up as nerve cells with their long thread-like projections appear, down which electrical signals can be transmitted.
Being able to access large numbers of the dopamine neurons in the lab should allow the study of all the biological processes that go wrong in Parkinson’s, giving greater insight into the causes of the disease.
"The brain is an inaccessible organ and you can't get bits of people's brain to study very easily," Dr. Richard Wade-Martins told BBC News. "But what we have here is a disease in a dish [where the cells] are just like Derek's brain cells but are accessible and can be produced in unlimited quantities."
Dr. Wade-Martins and colleagues now have samples from 20 different patients with Parkinson’s disease, all at different stages in the stem cell process. This gives a bank of samples for study.
The stem cell work is part of a bigger clinical study funded by Parkinson’s UK involving around 2000 people with Parkinson’s disease in the Thames Valley region.
The study is using a series of approaches – blood screens, gene sequencing, cell cultures, and MRI scans – to get a lot of clinical data about this set of patients to really probe the origins and causes of the condition.
"We’re interested in biomarkers that can be predictors of disease, whether that’s aberrant proteins in the blood or MRI imaging signatures," explains Dr. Wade-Martins.
"It’s not a clinical trial of a drug, nor are we using stem cells as a therapy. What we will get is a fantastically well-characterised cohort of people that should help us pinpoint disease mechanisms."