Genetic controller prepares immune system for diverse threats
An army of immune cells circulates the human body to protect against its potential foes—viruses, bacteria, cancer cells, and other invaders. Because the immune system cannot know what to expect, it must be prepared to fend off virtually any foreign pathogen it might encounter. Now, Howard Hughes Medical Institute scientists have identified a genetic regulator that controls the reshuffling of gene segments that immune cells use to manufacture billions of distinct antibodies and pathogen-recognizing receptors from a limited number of genes.
Mutating that regulatory region, the scientists have discovered, causes a cell to create an incorrect mishmash of antibody parts. The finding is important not only for understanding how antibodies are made, but for understanding how gene regulation can be coordinated across distant regions of the genome. The research was published on September 11, 2011, in the journal Nature.
HHMI investigator Frederick W. Alt of Children’s Hospital Boston, who has spent his career studying how the immune system generates diverse antibodies, says the discovery of this master regulator is the culmination of decades of work in his lab. “This site impacts every regulatory process that we’ve been studying for 30 years,” he explains.
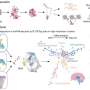
A typical antibody is a Y-shaped molecule made up of four chains: two identical short chains and two identical long – or heavy – chains. The part of antibodies that Alt focuses on, called the immunoglobulin heavy chain (IgH), is composed of three distinct segments: Variable (V), Diversity (D), and Joining (J). Hundreds of versions of the genes that encode these three segments are scattered throughout the genome, separated by long stretches of DNA. To prepare for antibody production, a developing immune cell stitches together one V segment, one D segment, and one J segment to form a gene that encodes a single heavy chain. Gene reshuffling creates about 100 billion possible combinations of the three segments. While each immune cell will generate many copies of the same antibody, the random selection of gene segments that takes place in each cell generates an assortment of antibodies to equip the immune system to recognize many molecules.
Alt had previously found that IgH formation always begins with the combination of a J and a D gene segment. It’s only later than a V is added. Because of this specific ordering, Alt suspected that genes that control the steps of so-called V(D)J recombination may lie in the area between the D and V segments. When his lab started to look more closely at the region, they saw tell-tale signs of genetic regulatory sequences.
“All these marks on the structure of the DNA said that this region had something active about it,” says Alt.
Now, he’s found one stretch of DNA that controls the timing of V(D)J recombination. Normally, Alt says, a protein binds to the site and forms loops of DNA that bring together the variable gene segments, but also act like a brick wall. The looped DNA before the V gene segments stops the cell from adding them to the chain before the J and D are combined. But when Alt’s lab mutated the site, V genes are stitched on too early. Moreover, the cell starts to favor V segments that that lie in closer proximity. This depletes the normal randomness of antibody formation. “By mutating this one site,” says Alt. “You lose the ability to make a diverse repertoire of antibodies.”
Alt suspects the region contains other regulatory elements, as well. There are many questions left to answer about how antibody diversity is generated.
“There are likely other factors binding this region that play a role in this process as well,” says Alt. “We don’t think we’ve finished figuring out how this works.”
Most of the known regulatory elements of genes are in the DNA directly flanking the gene. But in the case of antibodies, the many V, D, and J gene segments are so spread out that any regulatory elements must be acting across long distances. The newly discovered site only explains in part how this is accomplished. More work, Alt says, will reveal how the cell can control such spatially distant genes at once, ensuring that they are transcribed and combined properly.
“The immunoglobulin gene locus has always served as a pioneering place to look at gene regulation because it’s so complex and uses all these examples of regulation,” he says. “Enhancers were discovered there, as well as recombination mechanisms, and repair pathways. And now the immunoglobulin genes are providing us with a system to define how gene expression can be regulated over long distances.”