Scientists reveal surprising picture of how powerful antibody neutralizes HIV
Researchers at The Scripps Research Institute have uncovered the surprising details of how a powerful anti-HIV antibody grabs hold of the virus. The findings, published in Science Express on October 13, 2011, highlight a major vulnerability of HIV and suggest a new target for vaccine development.
"What's unexpected and unique about this antibody is that it not only attaches to the sugar coating of the virus but also reaches through to grab part of the virus's envelope protein," said the report's co-senior author Dennis Burton, a professor at The Scripps Research Institute and scientific director of the International AIDS Vaccine Initiative's (IAVI) Neutralizing Antibody Center, based on the Scripps Research La Jolla campus.
"We can now start to think about constructing mimics of these viral structures to use in candidate vaccines," said co-senior author Ian Wilson, who is Hansen Professor of Structural Biology and member of the Skaggs Institute for Chemical Biology at Scripps Research.
Other institutions in the United States, United Kingdom, Japan, and the Netherlands contributed to the research as part of an ongoing global HIV vaccine development effort.
Getting a Better Grip on HIV
Researchers from the current team recently isolated the new antibody and 16 others from the blood of HIV-infected volunteers, in work they reported online in the journal Nature on August 17, 2011. Since the 1990s, Burton, Wilson, and other researchers have been searching for such "broadly neutralizing" antibodies against HIV—antibodies that work against many of the various strains of the fast-mutating virus—and by now have found more than a dozen. PGT 128, the antibody described in the new report, can neutralize about 70 percent of globally circulating HIV strains by blocking their ability to infect cells. It also can do so much more potently—in other words, in smaller concentrations of antibody molecules—than any previously reported broadly neutralizing anti-HIV antibody.
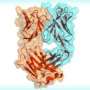
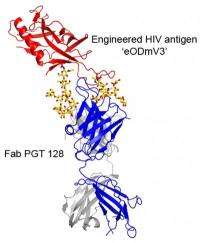
The new report illuminates why PGT 128 is so effective at neutralizing HIV. Using the Wilson lab's expertise in X-ray crystallography, Robert Pejchal, a research associate in the Wilson lab, determined the structure of PGT 128 joined to its binding site on molecular mockups of the virus, designed in part by Robyn Stanfield and Pejchal in the Wilson group and Bill Schief, now an IAVI principal scientist and associate professor at Scripps Research, and his group. With these structural data, and by experimentally mutating and altering the viral target site, they could see that PGT 128 works in part by binding to glycans on the viral surface.
Thickets of these sugars normally surround HIV's envelope protein, gp120, largely shielding it from attack by the immune system. Nevertheless, PGT 128 manages to bind to two closely spaced glycans, and at the same time reaches through the rest of the "glycan shield" to take hold of a small part of structure on gp120 known as the V3 loop. This penetration of the glycan shield by PGT 128 was also visualized by electron microscopy with a trimeric form of the gp120/gp41 envelope protein of HIV-1 by Reza Kayat and Andrew Ward of Scripps Research; this revealed that the PGT 128 epitope appears to be readily accessible on the virus.
"Both of these glycans appear in most HIV strains, which helps explain why PGT 128 is so broadly neutralizing," said Katie J. Doores, a research associate in the Burton lab who was one of the report's lead authors. PGT 128 also engages V3 by its backbone structure, which doesn't vary as much as other parts of the virus because it is required for infection.
PGT 128's extreme potency is harder to explain. The antibody binds to gp120 in a way that presumably disrupts its ability to lock onto human cells and infect them. Yet it doesn't bind to gp120 many times more tightly than other anti-HIV antibodies. The team's analysis hints that PGT 128 may be extraordinarily potent because it also binds two separate gp120 molecules, thus tying up not one but two cell-infecting structures. Other mechanisms may also be at work.
Toward an AIDS Vaccine
Researchers hope to use the knowledge of these antibodies' binding sites on HIV to develop vaccines that stimulate a long-term—perhaps lifetime—protective antibody response against those same vulnerable sites.
"We'll probably need multiple targets on the virus for a successful vaccine, but certainly PGT 128 shows us a very good target," said Burton.
Intriguingly, the basic motif of PGT 128's target may mark a general vulnerability for HIV. "Other research is also starting to suggest that you can grab onto two glycans and a beta strand and get very potent and broad neutralizing antibodies against HIV," Wilson said.