Gene therapy shows promise as hemophilia treatment in animal studies
For the first time, researchers have combined gene therapy and stem cell transplantation to successfully reverse the severe, crippling bleeding disorder hemophilia A in large animals, opening the door to the development of new therapies for human patients.
Researchers at Wake Forest Baptist Medical Center's Institute for Regenerative Medicine, collaborating with other institutions, report in Experimental Hematology that a single injection of genetically-modified adult stem cells in two sheep converted the severe disorder to a milder form. The journal is a publication of the Society for Hematology and Stem Cells
"A new approach to treating severe hemophilia is desperately needed," said lead author Christopher D. Porada, Ph.D., associate professor of regenerative medicine at Wake Forest Baptist. "About 75 percent of the world doesn't have access to the current treatment – therapy to replace missing clotting factors. This puts patients in most of the world at risk of severe and permanent disabilities."
Porada cautioned that challenges will need to be overcome before the treatment can be applied to humans, including that the sheep developed an immune response to the therapy that could decrease its effectiveness and duration.
There is currently no cure for the rare bleeding disorder hemophilia. People with this genetic disorder lack a protein, known as a clotting factor, needed for normal blood clotting. As a result, they may bleed for a longer time than others after an injury, as well as bleed internally, especially in joints such as the knees, ankles, and elbows. This bleeding can damage the organs and tissues and be life threatening. Even when life-threatening bleeds are prevented with replacement therapy, it doesn't prevent smaller bleeds within the joints that can cause pain and decreased mobility.
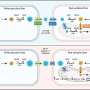
People with hemophilia A, the most common type, are missing clotting factor VIII. For the study, the researchers used a combined stem cell/gene therapy approach to increase levels of factor VIII produced by the animals.
The scientists first inserted a gene for factor VIII into engineered mesenchymal stem cells, a type of adult stem cell. The cells – acting as a carrier for the gene – were then injected into the abdominal cavity of the sheep. The scientists selected mesenchymal stem cells to carry the gene because they have the ability to migrate to sites of injury or inflammation.
In the treated animals, the cells migrated to the joints and stopped ongoing bleeding. In addition, all spontaneous bleeding events ceased, and the existing joint damage was completely reversed, restoring normal posture and gait to these crippled animals, and enabling them to resume a normal activity level.
However, a paradox of the treatment was that while the symptoms were eliminated, the sheep developed an immune response to factor VIII, suggesting that the treatment's effects would be reduced or shorter in duration. The scientists are currently working to learn why the immune response occurred and to develop strategies to prevent it.
"While preliminary, these findings could pave the way for a new therapy for hemophilia patients who experience debilitating bleeding in their joints," Porada said.