Ohio State researchers design a viral vector to treat a genetic form of blindness
Researchers at Ohio State University Medical Center and Nationwide Children's Hospital have developed a viral vector designed to deliver a gene into the eyes of people born with an inherited, progressive form of blindness that affects mainly males.
The vector is part of a clinical trial investigating the use of gene therapy to cure choroideremia, a disease that affects an estimated 100,000 people worldwide. The trial is being conducted by researchers at the University of Oxford in England.
The vector was designed by Dr. Matthew During, professor of molecular virology, immunology and medical genetics and of neuroscience and neurological surgery at Ohio State, in collaboration with Robert MacLaren, professor of ophthalmology at the University of Oxford, who also leads the trial.
Researcher Dr. K. Reed Clark, director of the Clinical Manufacturing Facility at the Center for Gene Therapy, Nationwide Children's Hospital, and his team produced the clinical-grade vector that is administered to patients in the trial.
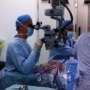
During, who is also a visiting professor of translational neuroscience at Oxford, was in the operating room during the pioneering surgery. "I and my colleagues are excited about contributing to this significant medical breakthrough," During says. "We have worked for many years to engineer and optimize viruses to safely deliver genes to humans, and the eye is an ideal target in many ways. The clinical vector manufacturing facility at Nationwide Children's Hospital is outstanding, and Dr. Clark and his team deserve congratulations for providing a clinical vector that for the first time offers these patients the possibility of an effective therapy."
During and his colleagues designed the viral vector to infect the light-sensitive photoreceptor cells that line the back of the eye and make up the retina. Choroideremia causes a degeneration of these photosensitive retinal cells and progressive blindness. The diagnosis is usually made in childhood and leads to blindness by around age 45
"This trial represents the first attempt to treat this disease and the first time that gene therapy has been directed towards the photoreceptor cells of the human retina," During says. "We believe it holds great promise for the treatment of other genetic causes of blindness such as retinitis pigmentosa."
The trial's 12 patients will be treated in one eye. It will take 24 months to know whether the gene-therapy treatment has stopped the degeneration. The trial builds on gene-therapy research performed in collaboration with Professor Miguel Seabra at Imperial College London, along with During and Clark at Ohio State.
"This disease has been recognized as an incurable form of blindness for over a hundred years," MacLaren says. "I cannot describe the excitement in thinking that we have designed a genetic treatment that could potentially stop it in its tracks with one single injection."