Trials for new ultrasound device
An Adelaide ultrasound device is set to reduce invasive treatments for women after childbirth, thanks to a collaboration arranged by the Flinders University-based Medical Device Partnering Program (MDPP).
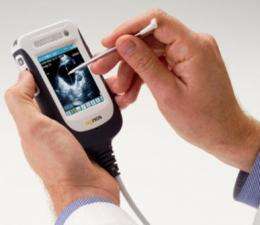
Clinical trials by the Department of Obstetrics and Gynaecology at Flinders Medical Centre will assess the performance of the world-leading handheld ultrasound device (pictured) engineered and manufactured by Adelaide company Signostics Limited.
Urinary retention is a serious problem for up to 15 per cent of women after birth, but current methods of urinary output measurement are subjective and can result in the unnecessary catheterization of patients, which increases the risk of urinary tract infections.
Accurate diagnosis remains vital, since a single episode of postpartum bladder overdistension can cause persistent urinary retention and irreversible damage.
The Signostics device has the potential to provide more objective and accurate measurements of residual bladder volume.
Co-founder and Chief Operating Officer of Signostics, Mr. Stewart Bartlett, said that the company has been looking for opportunities to assess and validate its ultrasound device.
“Before working with the MDPP, we found it very difficult to undertake research in SA, as we had no local research networks. Local evidence or references will assist with marketing our product internationally,” Mr. Bartlett said.
The new research collaboration will assist Signostics to enter new markets with their device, using the clinical evidence and validation of the effectiveness of their product in differing applications.
MDPP Director Professor Karen Reynolds said the program is all about bringing industry, researchers and end-users together to add value to South Australian medical device product development.
“It is important that we support our growing medical devices industry to maximize opportunities within South Australia,” Professor Reynolds said.
She said the real benefit of such collaborations is for patients, in this case women with postpartum urinary retention.
“If it is effective, the device could be instrumental in reducing the number of unnecessary catheterisations for women.”
MDPP are managing the process of the clinical trial and results will be finalized in the coming months.















