Head-to-head study in RA shows that abatacept has comparable efficacy to adalimumab
Data from one of the few head-to-head trials in rheumatoid arthritis (RA) presented today at EULAR 2012, the Annual Congress of the European League Against Rheumatism, demonstrates that at one year, 64.8% of patients receiving abatacept (Orencia) and 63.4% of patients receiving adalimumab (Humira) achieved ACR20.
The Phase IIIb AMPLE study (Abatacept Versus Adalimumab Comparison in Biologic-Naive RA Subjects with Background Methotrexate) was carried out in 646 biologic-naïve patients with active RA and inadequate response to methotrexate. At four weeks, 42.5% of patients in the abatacept group achieved ACR20 response versus 47.6% in the adalimumab group. This remained comparable until the end of year one. At 12 months, ACR50* response was similar between the two groups (46.2% in the abatacept group and 46% in the adalimumab group). ACR70 response was 29.2% versus 26.2% in the abatacept group and adalimumab groups respectively. These data show the similar time course of response. Inhibition of radiographic progression was also similar in both arms.
"There have been very few head-to-head trials in rheumatoid arthritis and, to date, there have been no randomised, controlled studies directly comparing the safety and efficacy of different biologic disease-modifying anti-rheumatic drugs (DMARDs) using the combination of a biologic medication and methotrexate which is the most commonly prescribed treatment approach in moderate to severe RA," said Dr. Michael Schiff, University of Colorado, USA and lead author of the study. "This study is a great leap forward for us and our patients as it shows there is another treatment option that is as effective and as safe as adalimumab."
The AMPLE study is a randomised, investigator-blinded study of 24 months, with a 12 month efficacy primary endpoint. Patients were stratified by disease activity and randomised to either 125mg subcutaneous abatacept (without an IV load) weekly or 40mg subcutaneous adalimumab bi-weekly, in combination with a stable dose of methotrexate. The primary endpoint of the trial was non-inferiority by ACR20 response at 12 months with a non-inferiority margin of 12%.
There was a similar rate of adverse events, serious adverse events, serious infections and malignancies in both groups.
More patients in the abatacept arm experienced autoimmune adverse events (3.1% versus 1.2%), but none were considered to be serious. There were also fewer discontinuations due to adverse events (2.5% versus 6.1%) and serious infections (0 versus 5 discontinuations) in the abatacept arm. Injection site reactions also occurred in fewer abatacept patients (3.8% versus 9.1%, 95% confidence interval: -5.37 (-9.13, -1.62) p=0.006).
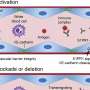
Abatacept (Orencia), which is produced by Bristol-Myers Squibb, is a first-in-class biologic which works to reduce co-stimulation of T-cells, which in turn reduces activation of other cells in the RA inflammatory process, thereby blocking the pain, inflammation and joint progression pathways in RA.
Adalimumab (Humira) is produced by Abbott and is a biologic TNF-blocker, or anti-TNF. Adalimumab works by binding to TNF-alpha receptors, thereby blocking the pain, inflammation and joint progression pathways in RA.
More information: Abstract Number: OP0022