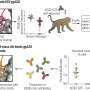
Under the right conditions, peptide blocks HIV infection at multiple points along the way
Human defensins, aptly named antimicrobial peptides, are made in immune system cells and epithelial cells (such as skin cells and cells that line the gut). One of these peptides, human neutrophil peptide 1, under certain circumstances hinders HIV infection, but exactly how it works remains unclear.
HIV entry into mature T-helper cells (cells essential to the immune system) proceeds by attachment of the virus to specific targets on T-helper cells, uptake of the virus, fusion of its envelope with the cell membranes, and release of the virus into the cells. In a forthcoming Journal of Biological Chemistry Paper of the Week, Gregory Melikyan at Emory University and colleagues investigated the ability of human neutrophil peptide 1 to impede each step of this process.
Using model cell lines, Melikyan's group showed that human neutrophil peptide 1 effectively prevented HIV entry into cells in multiple ways. First, human neutrophil peptide 1 reduced the number of specific targets on the cells available for HIV attachment. Second, this defensin also bound to specific targets on both the HIV envelope and the cells, preventing early and late stages of HIV-cell fusion. Finally, human neutrophil peptide 1 prevented HIV uptake into the cells without compromising the general ability of the cells to engulf other molecules.
While human neutrophil peptide 1 hinders HIV entry into cells under these lab conditions, it does not do so as effectively in the presence of serum -- meaning that it may not be as successful at blocking HIV in our bodies. But Melikyan's team showed that human neutrophil peptide 1 remained attached to its specific targets in the presence of serum, despite its reduced efficacy. Their work suggests that the structure of human neutrophil peptide 1 is important for its anti-HIV activity, and they propose that serum may interfere with the ability of this defensin to form complexes, reducing its ability to block HIV.
"Our work provides new insights into the ability of defensins to recognize and neutralize diverse pathogens, including HIV," Melikyan says. This research reveals that human neutrophil peptide 1 can bind various viral and cellular targets and that a previously unappreciated feature is essential for its anti-HIV activity, possibly its propensity to form large complexes, Melikyan explains.
The team's findings suggest a new avenue of research for combatting HIV and viruses that infiltrate cells in a similar manner.
More information: "Multifaceted mechanisms of HIV-1 entry inhibition by human alpha-defensin" by Lusine H. Demirkhanyan, Mariana Marin, Sergi Padilla-Parra, Changyou Zhan, Kosuke Miyauchi, Maikha Jean-Baptiste, Gennadiy Novitskiy, Wuyuan Lu, and Gregory B. Melikyan (to be published in the Aug. 17 issue of the Journal of Biological Chemistry and currently online as a Paper in Press at www.jbc.org/content/early/2012 … M112.375949.full.pdf )