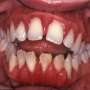
Gum disease: The most common form of bone loss
On July 19, the Canadian Institutes of Health Research announced it will be funding the work of three research teams investigating bone health. The University of Toronto’s Dr. Michael Glogauer, Associate Professor with the Faculty of Dentistry, heads one of those teams. A clinician scientist whose post-doctoral work at Harvard focussed on neutrophils – white blood cells that help fight infection and inflammation - Glogauer spoke with U of T News about seeking new ways to identify bone loss in gum disease.
When we hear the words “bone loss”, osteoporosis comes to mind. What does bone loss have to do with gum disease?
The most common form of bone loss is not osteoarthritis or osteoporosis – it’s periodontal or gum disease. And with three-quarters of the population expected to experience it you can imagine the costs associated with it.
Because it’s painless most people don’t realize it’s going on until it’s too late. By the time they decide to go to the dentist or end up at the periodontal gum specialist, they’ve actually lost significant amounts of bone. They may even lose their teeth and require implant placement or dentures.
What are the symptoms?
Bleeding gums is a sign but it will come and go – periodontal disease is episodic, like all inflammatory diseases. There will be periods where it’s very active and periods where it’s quiet – and a lot of people will just ignore it.
Eventually, teeth will start to shift, to move around. There will be pain on chewing or teeth will actually start to become loose. And when the teeth become loose it’s almost too late at that point.
How will your research help?
What we’re trying to do is identify novel diagnostic biomarkers that will allow us to do a couple of things. First, we want to develop diagnostic tests like a rinse which patients can use at home or in a dental office to identify if you have disease at the very early stages, before bone loss starts to occur.
Second, there are some patients who have very severe forms of the disease and are very resistant to treatment. We want to try to identify markers to show which patients are at very high risk for the aggressive forms of the disease so they can be treated more aggressively than the standard patient.
With this grant, we’ll be collecting fluid from the patient’s mouth and around the teeth and carrying out high throughput screening and sophisticated analysis of these fluids in order to identify possible novel biomarkers.
Is this all taking place at U of T?
This money will allow us to take our research to the next level by creating a collaborative team. Our team includes Dr. Christopher Overall at the University of British Columbia. He’s one of the world’s leading experts on doing a specific type of proteomic analysis. Using mass spectrometry, we’ll be able to look, not just at proteins in the fluids, but also at proteases that modify these proteins and change their functions. We’re trying to see if these fine-tuned proteins will serve as more significant or pertinent biomarkers.
There are three of us here in Toronto – Professor Sergio Grinstein, Professor Christopher McCulloch and myself. The fifth member is Dr. Deborah Mathews working out of Dalhousie so we really have a cross-Canada team, all the way from Dalhousie to UBC.
Drs Mathews, McCulloch and I are going to also focus on the knowledge translation aspect of the project.
It’s not enough to just identify the biomarkers and come up with the test. Right from the beginning we want to make contact with industry, with the various patient groups and clinician provider groups to try to come up with the best way to formulate the test and the best way to get that into the clinics so that patients can start using it as soon as it becomes available. That’s knowledge translation.
Earlier this year you also received one of U of T’s Connaught Innovation Awards for something called a colourmetric rinse test. Is that related to bone health?
The colormetric rinse test measures the oral inflammatory load – basically it’s a way of telling you that a patient has significant inflammation in their gums, and that patient is in need of treatment.
The key marker that we’re using there is a type of white blood cell called a neutrophil. It’s an excellent tool for letting us know that the patient has inflammation and the severity of the inflammation we can use to make certain the treatment is successful. What we’re trying to do with this grant, going forward is to try to catch things even earlier in the process. And these biomarkers hopefully can also tell us which patients are not going to respond well to the treatment.
The more aggressive forms, which probably happen in less than five percent of cases, patients come in and they’ve got excellent hygiene, they’re doing everything they should, visiting the dentist every three to six months, and they’re still breaking down, still going to lose their teeth. So we want to identify these patients early on.
We also want to try to find some of the genes associated with the aggressive bone loss in these patients in hopes that, by understanding the genetics we can improve treatment as well.
Could your work help with other forms of bone loss, or other conditions affected by inflammation?
There’s a consensus now that you can almost pick any disease, and it’s associated with inflammation. Inflammation has a hand in everything - osteoporosis, cardiovascular disease, cancer. Pick a condition and it’s affected by inflammation.
Periodontal disease is the prototypic, osteo-immune disease, where the inflammatory system is impacting on bone and causing bone to be lost. So we believe that the markers we find in the mouth will be very applicable for diseases such as arthritis. We believe that the mechanisms are almost identical.
This will be an excellent way to take the knowledge we find and test it out for other conditions.
At any time you have between eight and 12 students working your lab – dentistry students or medical students?
I’m a dentist but half the stuff in my lab is not really dental focused. We use transgenic mouse models so we can really understand the immune system and osteoclasts (cells that break down bone) and the osteo-immune connection, take what we’re doing fundamentally at the bench with mice and cells, and then look at what’s happening with patients.
We have masters students, PhDs, one or two post-docs and particularly during the summer we usually have about four to six undergrads working in the lab. About two-thirds are usually on the dental side but we have medical students as well.
But it’s not just about training doctors and dentists. My goal is to try to create clinician scientists. I’m trying to turn people on to research because I think it’s critically important – we need smart people to help us figure out the answers to the important questions we have.