Seattle researchers to engineer kidney tissue chip for predicting drug safety
(Medical Xpress) -- Seattle researchers will be part of the new federal initiative to engineer 3-dimensional chips containing living cells and tissues that imitate the structure and function of human organs. These tissue chips will be used for drug safety testing.
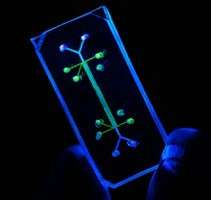
Tissue chips merge techniques from the computer industry with those from bioengineering by combining miniature models of living organ tissues onto a transparent microchip. Ranging in size from a coin to a house key, the chips are lined with living cells and contain features designed to replicate the complex biological function of a specific organ.
The Seattle team will design, implement and test a tissue-engineered human kidney microphysiological system. Kidneys, which clear the blood of waste products, are among the sensitive organs that can be damaged by certain medications, environmental toxins or an excess of natural substances produced by the body.
The Seattle project, announced July 24, is led by Dr. Jonathan Himmelfarb, University of Washington professor of medicine in the Department of Medicine, Division of Nephrology, and director of the Kidney Research Institute. The project team consists of physicians, bioengineers, pharmacists, environmental health researchers, and pharmaceutical developers from the UW schools of medicine, public health and pharmacy, and the College of Engineering. The amount and years of of funding to the UW are yet to be announced, pending Notice of Grant Award. Overall, the national initiative is budgeted at $70 million.
Their project proposal is one of 17 nationwide funded in a recent round of awards from the new National Center for Advancing Translational Sciences of the National Institutes of Health. This grant program, a collaboration with the Defense Advanced Research Projects Agency and the U.S. Food and Drug Administration, was created to improve methods for predicting whether newly developed drugs will be safe in humans.
The goal is to develop human tissue chips that simulate the structure and function of human organs, such the lung, heart, liver, and kidneys. Scientists could then use these tissue chips to test drug candidates and predict their safety before the next step, human drug studies. This approach is expected be more rapid and cost effective than those currently available.
The NIH pointed to studies that show that more than 30 percent of promising medications have failed in human clinical trials because the drugs were found to be toxic, despite pre-clinical studies in animal models. Tissue chips may offer more accurate predictions of the side effects of potential therapeutic agents because they contain human cells.
Ten of the 17 new awards will support studies to design 3-dimensional cellular microsystems that represent different human organs. These bioengineered devices will produce relevant physiological functions and will reflect the complexity and diversity of living organs, including genetic differences, disease complexity and pharmacological responses. The additional seven National Center for Advancing Translational Sciences awards will explore the potential of stem cells and progenitor cells to form the many cell types that make up the architecture of complex organs. These could be a source of cells to populate tissue chips.
Himmelfarb and his colleagues propose to create a tiny, 3-dimensional lab device containing engineered biological tissues that will perform certain actions of a living human kidney. The system would evaluate the uptake, breakdown and elimination of potentially toxic substances, and predict the rate for these chemical reactions. The system might also help assess kidney injury from infections disease organisms and from toxins, both those introduced into the body and those produced by the body.
The micro-model of kidney physiology will also feature two parallel structures – small blood vessels and the surface lining of the renal tubules. This aspect of the device will enable researchers to study the complex interactions between these two structures, which are normally in intimate association inside each of the functional units of the kidney, the nephrons.
In addition to Himmelfarb, the UW project team includes Jeremy Duffield from the Department of Medicine, Division of Nephrology; Ying Zheng from the Department of Bioengineering; Ken Thummel and Joanne Wang from the Department of Pharmaceutics; David Eaton, of the Department of Environmental and Occupational Health and the UW Center for Ecogentics and Environmental Health, and Danny Shen from the Department of Pharmacy.
Nortis Inc., a bioengineering start-up company funded through the UW’s Center for Commercialization, will also be a partner in the project. Thomas Neumann is president and CEO of Nortis. Project plans include using the Life Sciences Development Fund-supported Washington Phenotype Biospecimen Resource to obtain kidney tissue specimens for the project.