Shining light on amyloid protein nanostructures

Scientists from the MESA+ and MIRA research institutes at the University of Twente have developed a new method to gain insight into the composition of large macromolecular protein assemblies. Their method allows the determination of the composition of potentially toxic amyloid protein assemblies involved in many human neurodegenerative diseases. The research team recently published their results in the leading scientific journal Angewandte Chemie, which highlighted this work on the cover of the journal.
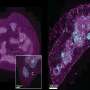
Many human neurodegenerative diseases, such as Parkinson’s disease, Alzheimer’s disease, or Huntington’s disease, are the result of protein misfolding. As a result of this misfolding, the monomeric proteins can aggregate and form small protein clumps, so-called oligomers. These oligomers are thought to be key players in the disease process. However, obtaining information about the exact composition, that is, the number of monomeric proteins that form one oligomer, remains very challenging, while this is essential information for understanding the disease process.
Single-molecule photobleaching and sub-stoichiometric labeling
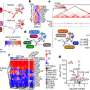
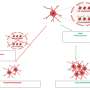
The research team has developed a new method to determine the composition of these oligomers. Combining single-molecule photobleaching techniques with sub-stoichiometric fluorophore labeling gave insights into the number of monomers that form a protein oligomer. Single-molecule photobleaching uses ultrasensitive fluorescence microscopy to observe the successive photodestruction of fluorophores within one oligomer. To make this method suitable for large oligomers, the researchers have extended this method to be used in combination with sub-stoichiometric labeling, in which only a fraction of the monomeric proteins contain a fluorescent label, and statistical analysis of the data.
Alpha synuclein oligomers
The newly developed method can be applied in general to large macromolecular protein assemblies. The research team has focused on the neuronal protein alpha-synuclein, which plays a critical role in the onset and progression of Parkinson’s disease. They showed that alpha-synuclein oligomers prepared by a specific protocol are present as a single-well defined species consisting of 31 monomers per oligomer.
More information: Full article: onlinelibrary.wiley.com/doi/10 … 2/anie.201200813/pdf