Combination therapy delivers one-two punch to skin cancer, boosting anti-tumor activity
(Medical Xpress) -- Treating metastatic melanoma by combining immunotherapy with a drug that inhibits the cancer-spreading activity of a common gene mutation significantly increased survival times in an animal model, according to a study by researchers at UCLA's Jonsson Comprehensive Cancer Center.
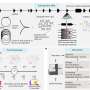
In the study, animals that received a combination of the recently approved BRAF inhibitor Zelboraf and an engineered T-cell immunotherapy had better tumor responses and lived more than twice as long as those getting the BRAF inhibitor or immunotherapy alone. The findings provide strong support for testing the combination therapy in human clinical trials, which Jonsson Cancer Center researchers hope to launch within two years.
About 50 percent of patients with metastatic melanoma — some 4,000 people a year — have the BRAF mutation and can be treated with Zelboraf. More than 50 percent of them respond well to the drug, but the responses usually last only a few months. With immunotherapy, fewer patients respond, but the responses are more durable.
By pairing these therapies in a one–two punch, researchers hope to maintain the high response rates associated with Zelboraf and combine them with the longer disease-free progression times seen with immunotherapy, said the study's first author, Dr. Richard Koya, a Jonsson Cancer Center scientist and an assistant professor of surgical oncology at UCLA.
"The idea was to target two different aspects of anti-cancer biology, hitting the tumor cells themselves with the BRAF inhibitor and adding in T cells educated to induce a specific anti-tumor immune response," Koya said. "The results we saw in this study were very promising."
The findings of the two-year study were published Aug. 15 in the peer-reviewed journal Cancer Research.
The researchers also found that the BRAF inhibitor helped boost the power of the immunotherapy, creating a greater combination effect, said senior study author Dr. Antoni Ribas, a Jonsson Cancer Center scientist and UCLA professor of hematology–oncology.
"We found that both treatments were more effective when administered together, and we were surprised to see that a drug that should only be targeting the BRAF-mutant cancer cells was also having a beneficial effect on the T cells," Ribas said.
In the immunotherapy technique, called adoptive T-cell transfer (ACT), lymphocytes are genetically engineered to express a receptor that recognizes melanoma cells, creating an army of immune cells that attack the cancer. The lymphocytes are modified genetically to become specific to the melanoma cells and are injected into the body.
The study was done using a model based on unique cell lines developed at UCLA. Previously, no implantable BRAF mutation–driven melanoma model that was able to grow progressively in mice with fully competent immune systems was available.
It is vital to develop new drugs to treat metastatic melanoma, as few options are available for patients, the researchers said. Zelboraf works well, but most patients eventually relapse.
"This is a patient population that we are not able to cure," Koya said. "With what we have now, we are just prolonging their lives. We need to have more options, and we hope this combination therapy proves to be an effective alternative."
About 70,000 new cases of melanoma are diagnosed each year in the United States. Of those, 8,000 people will die of the disease.
"In conclusion, combined therapy with the BRAF-specific inhibitor Zelboraf and T cell receptor engineered adoptive cell transfer resulted in superior anti-tumor effects," the study states. "Although the absolute number of T cells infiltrating the tumor was not increased by Zelboraf, the combination increased the functionality of antigen-specific T lymphocytes. Therefore, our studies support the clinical testing of combinations of BRAF targeted therapy and immunotherapy for patients with advanced melanoma."