Researchers use blood testing to predict level of enzymes that facilitate disease progression

Predicting how atherosclerosis, osteoporosis or cancer will progress or respond to drugs in individual patients is difficult. In a new study, researchers took another step toward that goal by developing a technique able to predict from a blood sample the amount of cathepsins—protein-degrading enzymes known to accelerate these diseases—a specific person would produce.
This patient-specific information may be helpful in developing personalized approaches to treat these tissue-destructive diseases.
"We measured significant variability in the amount of cathepsins produced by blood samples we collected from healthy individuals, which may indicate that a one-size-fits-all approach of administering cathepsin inhibitors may not be the best strategy for all patients with these conditions," said Manu Platt, an assistant professor in the Wallace H. Coulter Department of Biomedical Engineering at Georgia Tech and Emory University.
The study was published online on Oct. 19, 2012 in the journal Integrative Biology. This work was supported by the National Institutes of Health, Georgia Cancer Coalition, Atlanta Clinical and Translational Science Institute, and the Emory/Georgia Tech Regenerative Engineering and Medicine Center.
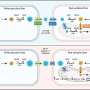
Platt and graduate student Keon-Young Park collected blood samples from 14 healthy individuals, removed white blood cells called monocytes from the samples and stimulated those cells with certain molecules so that they would become macrophages or osteoclasts in the laboratory. By doing this, the researchers recreated what happens in the body—monocytes receive these cues from damaged tissue, leave the blood, and become macrophages or osteoclasts, which are known to contribute to tissue changes that occur in atherosclerosis, cancer and osteoporosis.
Then the researchers developed a model that used patient-varying kinase signals collected from the macrophages or osteoclasts to predict patient-specific activity of four cathepsins: K, L, S and V.
"Kinases are enzymes that integrate stimuli from different soluble, cellular and physical cues to generate specific cellular responses," explained Platt, who is also a Georgia Cancer Coalition Distinguished Cancer Scholar. "By using a systems biology approach to link cell differentiation cues and responses through integration of signals at the kinase level, we were able to mathematically predict relative amounts of cathepsin activity and distinguish which blood donors exhibited greater cathepsin activity compared to others."
Predictability for all cathepsins ranged from 90 to 95 percent for both macrophages and osteoclasts, despite a range in the level of each cathepsin among the blood samples tested.
"We were pleased with the results because our model achieved very high predictability from a simple blood draw and overcame the challenge of incorporating the complex, unknown cues from individual patients' unique genetic and biochemical backgrounds," said Platt.
According to Platt, the next step will be to assess the model's ability to predict cathepsin activity using blood samples from individuals with the diseases of interest: atherosclerosis, osteoporosis or cancer.
"Our ultimate goal is to create an assay that will inform a clinician whether an individual's case of cancer or other tissue-destructive disease will be very aggressive from the moment that individual is diagnosed, which will enable the clinician to develop and begin the best personalized treatment plan immediately," added Platt.
More information: Park, Keon-Young et al., "Patient specific proteolytic activity of monocyte-derived macrophages and osteoclasts predicted with temporal kinase activation states during differentiation," Integrative Biology (2012): dx.doi.org/10.1039/C2IB20197F