Study shows key enzyme missing from aggressive form of breast cancer
A groundbreaking new study led by the University of Kentucky Markey Cancer Center's Dr. Peter Zhou found that triple-negative breast cancer cells are missing a key enzyme that other cancer cells contain—providing insight into potential therapeutic targets to treat the aggressive cancer. Zhou's study is unique in that his lab is the only one in the country to specifically study the metabolic process of triple-negative breast cancer cells.
Normally, all cells—including cancerous cells—use glucose to initiate the process of making Adenosine-5'-triphosphate (ATP) for fuel to carry out essential functions. This process, called glycolysis, leads to other processes that use oxygen to make higher quantities of ATP—but solid tumor cells, which have little access to oxygen, are forced to rely almost exclusively on aerobic glycolysis for survival.
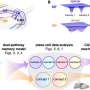
Zhou's study, published in Cancer Cell, showed that the powerful transcription factor complex Snail-G9a-Dnmt1 is over-expressed in triple-negative breast cancer, inhibiting the enzyme 1,6-bisphosphate (FBP1). The loss of this enzyme shuts down the glucose anabolic pathway and promotes the glucose catabolic pathway, leading to a large amount of glucose entering the tumor cells and thus "feeding" the aggressive cancer. This metabolic switch empowers the triple-negative breast cancer cells to suck more glucose from the body, increasing macromolecule biosynthesis in tumor cells and maintaining ATP production despite a dearth of nutrients and an oxygen-free environment.
Triple-negative breast cancer is the most deadly subtype of breast cancer, and tends to occur in women at a younger age. This subtype of breast cancer has poor clinical outcomes due to the early metastasis of tumor cells, resistance to chemotherapy, and the lack of specific drugs that target it. Identifying this change in the cancer's metabolic process provides major insight into developing drugs to target the disease, Zhou says.
"These findings present significant insights regarding the development and progression of triple-negative breast cancer," said Zhou, associate professor of molecular and cellular biochemistry at UK. "They indicate that targeting the metabolic alteration will lead to an effective approach for treating this deadly disease."
Zhou's research was aided by the team in the Free Radical Biology in Cancer Shared Resource Facility (FRBC) of the Markey Cancer Center, directed by Dr. Allan Butterfield. The FRBC used an instrument called the Seahorse XF-96 Flux Analyzer to test and confirm the predictions of Zhou's findings in triple-negative breast cancer.
"The significance of this study rests in proving that triple negative breast cancer cells utilize glycolysis for survival and growth," Butterfield said. "The FRBC will assist Dr. Zhou in furthering this exciting research, potentially helping to identify key proteins in triple negative breast cancer cells that are expressed or modified differently than in control cells. Such knowledge may lead to new insights to potential approaches to treat this aggressive cancer."