From shape-shifting to therapy
(Medical Xpress)—The latest research into the intricate processes that let substances into and out of cells will help to lay the foundations for the next generation of therapies for major diseases.
A team based at the University of Cambridge's Department of Pharmacology has developed a means of tracking – and thus understanding – the processes by which multi-drug transporters deflect and eject substances from cells. Their work will form the basis for the development of new therapies to treat cancers and infectious diseases.
Each cell in our bodies – and every cell of every organism from bacteria upwards – is surrounded by a fatty layer called the cell membrane. This membrane acts as a molecular sieve that enables entry of vital nutrients, such as sugars, into the cell and allows exit of the garbage. The traffic of substances across the membrane is carried out by tiny molecular machines, known as transport proteins. These transport proteins ensure a healthy environment in the cell's interior, so that cells can live and make more cells.
But the picture is much more complex as there are several types of transport proteins. While most transporters are specialists and welcome particular substances, some others eject an extraordinarily broad range of substances from the cell. These so-called 'multi-drug transporters' are essential defense systems in all organisms ranging from bacteria to humans. They prevent the entry into the cell of toxic substances that are produced by our bodies or released by microorganisms competing for space and nutrients.
"As transport proteins, multi-drug transporters are responsible for producing chemotherapy resistance in cancers and pathogenic micro-organisms. When toxic drugs are used as part of anticancer and antimicrobial therapies, multi-drug transporters can impair the treatment of life-threatening disease," said Dr Hendrik van Veen at Cambridge's Department of Pharmacology.
An understanding of how multidrug transporters work is essential to getting a better grasp both of the roles of multi-drug transporters in the healthy functioning of cells, and how they can be stopped when things go wrong – for example when malicious cells invade our bodies.
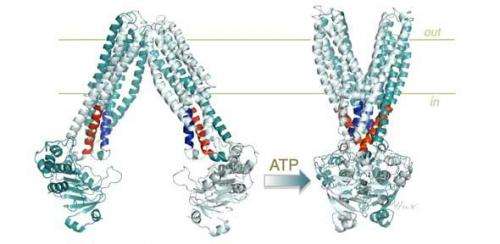
Scientists have proposed since the 1950s that transport proteins work by switching their three-dimensional shape, using metabolic energy as fuel to alter their conformation in order to 'flip' molecules from inside the barrier of the cell membrane to the outside. However, the exact molecular basis of this switching has baffled researchers for some time.
Dr Hendrik van Veen and colleagues investigated the mechanism of molecular shape-shifting of a multi-drug transporter that uses the energy-carrying molecule ATP (adenosine triphosphate). They used genetic engineering to tweak a critical part of the transporter – a group of four helical structures embedded in the molecule (referred to as the tetrahelix bundle) that seems to act as a 'spring-lock' for the larger molecular structure of the protein.
He said: "Multidrug transporters operate by a mechanism in which parts of their structure responsible for binding of toxic drugs are first exposed to the inside of the cell, where they bind the drug, and then to the outside of the cell, where they release it. This mechanism relies on two defined shapes of the transporter, an inward-facing shape and an outward-facing shape. On the basis of three-dimensional structures of the transporter, we postulated that the tetrahelix bundle would be important for this shape-shifting."
By manipulating the sections of DNA that control the architecture of the multi-drug transporter, the researchers were able to produce bacteria that made altered versions of the transport protein. The researchers then built the modified proteins into 'inside-out'-oriented bubbles of cell membrane in which the transporters import the drug from the outside, thus making it easier to follow the transport process.
Dr van Veen and his team were able to show that both transport and the associated changes in protein conformation were greatly impaired by the structural alterations in the tetrahelix bundle, while other features, such as the transporter's ability to bind ATP and drugs, were not significantly affected. Because the activity of multi-drug transporters is critical in preventing the entry of anticancer agents and antimicrobial drugs into cells, this research lays the foundation for the development of agents that will block the 'spring-lock' of the transporter, thus rendering it powerless.
"Tetrahelix bundles are shared by all ATP-dependent multi-drug transport proteins and are essential for their activity. To use a metaphor, we're working on the development of sticks to put in the spokes to stop these wheels from turning, and the use of these sticks in a next generation of anticancer and antimicrobial therapies that will tackle the development of drug resistance," said Dr van Veen.