April 19, 2013 report
Turning back the clock on regeneration in neurons
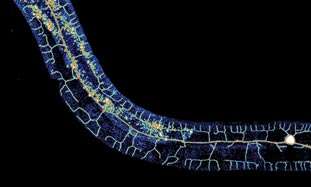
(Medical Xpress)—When minor wounds heal, the fine nerve endings that sense touch, or control sweating, are usually able to regrow. Like many processes in the body, the ability to regenerate new tissues changes throughout the lifecycle, typically diminishing with age. To investigate the molecular details of regeneration, the nervous system of the worm, C. Elegans, is ideal because its entire blueprint—the connectome—is available. The close-knit cadre of researchers who study C. elegans are the true veterinarians of neuroscience in that they command nearly every tool in the field to study this microcosm of biology. Publishing today in Science, a group of these researchers has uncovered a genetic circuit that regulates the regrowth of axons after they are experimentally cut with a laser. While the integrity of these mechanisms insures stability in the adult nervous system, manipulation of them could allow insults to the system to be restored to normal function.
In order to develop properly in the first place, the expression of the genes controlling tissue construction proceeds in a choreographed rhythm, with each having its proper time and place. Once the organism has developed, many of these genes are decommissioned, or their cycles of expression dephased. Sometimes two components that act together in the larval stage, oppose each other in the adult. Two players in this genetic tit-for-tat, lin-41 and let-7, have previously been found to act as timers during these transitions. The researchers in the study described here, stumbled upon this particular circuit while they were looking at the effect of yet another gene, alg-1, on axon regeneration. Specifically, they had found that worms with a mutant form of alg-1, could regenerate certain axons up to 2.5 times longer than the axons of normal adult worms.
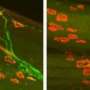
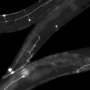
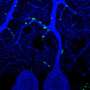
One particular sensory neuron, the AVM (anterior ventral microtubule) neuron, has a clearly defined axon that can regrow in larva, in not in adults. This strangely-named neuron has an even stranger subcellular feature. Its dendrites, in addition to the axon, are filled with a unique kind of microtubule, one that is composed of 15 protofilaments. Most mammals use a microtubule form-factor specifically made from 13 protofilaments, but many invertebrates use anywhere from 10 to 15. The avm neuron is also unique in that is one of just a few neurons that migrates to an asymmetric position in the body of the worm—it has no counterpart on the opposite side.
The AVM neuron shows clear expression not only the alg-1 gene, but also another factor regulated by alg-1 known as let-7. The researchers were able to show that let-7 is responsible for inhibiting adult regrowth in the AVM neuron. Inhibiting let-7 directly, or alternatively, increasing the level of its reciprocal inhibitor, lin-41, completely restored the regeneration capabilities of the larval axons. From this they conclude that cyclic interactions between let-7 and lin-41 are a general strategy used not only in determining cell fate in development, but also in controlling axon regeneration.
Expression of let-7 was controlled by using a version of the gene which is temperature-sensitive. The particular allele used has normal activity at 15 degrees C, but can be completely turned off at 20 degrees C. The actual product of the let-7 gene is ultimately not a protein, but one of a class of newly-discovered regulators known as microRNAs. The full functionality of microRNAs has yet to be completely defined, but they seem to be able to regulate proteins, DNA, and mRNA.
The researchers were also partial to speculation as to why the organism appears to take pains to inhibit regrowth in the adult. Axotomy by laser may not have been a primary selection criteria during the evolution of the worm, but some ability for tissue repair would be important in the life of a worm. In the greater scheme of things, it would seem that loss of certain capabilities in the adult, may be a small price to pay for the greater stability of connections that may come along with it.
We recently reported on a study in mice, which demonstrated that mature brains continue to remodel their fine structure throughout the entire life of the organism. Mammalian axons have the further complication that while myelination is required to conduct signals over appreciable distances, it can also be an impediment to regrowth. For axons that have been compromised by trauma, or through developmental fault, turning back the clock on a few genes may be only part of the puzzle.
More information: Developmental Decline in Neuronal Regeneration by the Progressive Change of Two Intrinsic Timers, Science 19 April 2013: Vol. 340 no. 6130 pp. 372-376. DOI: 10.1126/science.1231321
ABSTRACT
Like mammalian neurons, Caenorhabditis elegans neurons lose axon regeneration ability as they age, but it is not known why. Here, we report that let-7 contributes to a developmental decline in anterior ventral microtubule (AVM) axon regeneration. In older AVM axons, let-7 inhibits regeneration by down-regulating LIN-41, an important AVM axon regeneration–promoting factor. Whereas let-7 inhibits lin-41 expression in older neurons through the lin-41 3′ untranslated region, lin-41 inhibits let-7 expression in younger neurons through Argonaute ALG-1. This reciprocal inhibition ensures that axon regeneration is inhibited only in older neurons. These findings show that a let-7–lin-41 regulatory circuit, which was previously shown to control timing of events in mitotic stem cell lineages, is reutilized in postmitotic neurons to control postdifferentiation events.
© 2013 Medical Xpress