ECO: Distilled water doesn't up resting energy expenditure

(HealthDay)—Drinking 500 ml of purified water is not associated with increases in resting energy expenditure (REE), according to a study presented at the annual meeting of the European Congress on Obesity, held from May 12 to 15 in Liverpool, U.K.
Noting that studies report conflicting water-induced thermogenesis results, Nathalie Charrière, and colleagues from the University of Fribourg in Switzerland, conducted a study among 16 young men (aged 20 to 30 years) in whom REE was measured by ventilated hood indirect calorimetry before and for two hours after drinking 500 ml of water (kept at 21 degrees Celsius).
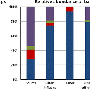
The researchers found that there was no significant increase seen after drinking 500 ml of purified (distilled) water (1.3 percent), whereas there was a significant but modest increase (5.8 percent) in REE after drinking 500 ml of bottled water (rich in calcium, magnesium, sodium, sulphate, and bicarbonate). Drinking bottled water plus ingestion of capsules of sodium bicarbonate (equivalent to or double that in bottled water) did not result in a significant difference in REE.
"Our results suggest that drinking 500 ml of purified water has little or no effect on REE," Charrière and colleagues conclude. "Whether the presence of minerals or salts (other than bicarbonates) and/or sensorial effects may explain differential thermogenic responses to water remains to be investigated."
More information: More Information
Health News
Copyright © 2013