Injecting iron supplement lets scientists track transplanted stem cells
A new, noninvasive technique for tracking stem cells after transplantation—developed by a cross-disciplinary team of radiologists, chemists, statisticians and materials scientists at the Stanford University School of Medicine—could help surgeons determine whether a procedure to repair injured or worn-out knees is successful.
The technique, described in a study to be published online July 12 in Radiology, relies on an imaging agent already approved by the U.S. Food and Drug Administration for an entirely different purpose: anemia treatment. Although this study used rodents, the approach is likely to be adapted for use in humans this fall as part of a clinical trial in which mesenchymal stem cells will be delivered to the site of patients' knee injuries. Mesenchymal stem cells are capable of differentiating into bone and cartilage, as well as muscle, fat and tendon, but not into the other cell types that populate the body.
Every year, arthritis accounts for 44 million outpatient visits and 700,000 knee-replacement procedures. But the early repair of cartilage defects in young patients may prevent further deterioration of the joint and the need for knee replacement later in life, said the study's senior author, Heike Daldrup-Link, MD, PhD, an associate professor of radiology and clinician who splits her time between research and treating pediatric patients.
Mesenchymal stem cells have been used with some success in cartilage-repair procedures. "These cells can be easily derived from bone marrow of patients who are going to undergo the knee-repair procedure," said Daldrup-Link, a member of the Molecular Imaging Program at Stanford. "And they can differentiate into the real-life tissues that compose our joints. But here, too, things can go wrong. The newly transferred cells might fail to engraft, or die. They might migrate away. They could develop into tissues other than cartilage, most commonly fibrous scar tissue."
Relatively few transplanted cells go the distance. The ability to monitor the cells' engraftment after they are deposited at a patient's knee-injury site is therefore essential. With the new technique, magnetic resonance imaging can visualize stem cells for several weeks after they have been implanted, giving orthopaedic surgeons a better sense of whether the transplantation was successful.
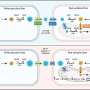
Until now, the only ways of labeling mesenchymal stem cells so that they could be noninvasively imaged have required their manipulation in the laboratory. Upon extraction, the delicate cells have to be given to lab personnel, incubated with contrast agents, spun in a centrifuge and washed and returned to the surgeons, who then transplant the cells into a patient.
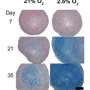
The new technique involves labeling the cells before extraction, while they reside in the donor's bone marrow. For the study, lead authors Aman Khurana, MD, a postdoctoral scholar, and Fanny Chapelin, a research associate, injected ferumoxytol, an FDA-licensed anemia treatment composed of iron-oxide nanoparticles, into rats prior to extracting bone marrow from them. Then, after enriching the mixture for mesenchymal stem cells, the investigators injected it into the sites of knee injuries in recipient rats. They followed the implanted cells' progress for up to four weeks, comparing the results with those obtained both from cells labeled in laboratory dishes and from unlabeled cells.
Daldrup-Link and others previously have used ferumoxytol for stem-cell labeling in a dish. However, mesenchymal stem cells in a laboratory dish take up very little of this substance. Interestingly, the researchers showed in a series of experiments that, ensconced in donor rats' bone marrow, the same cells are avid ferumoxytol absorbers. Even several weeks after transplantation into the recipient rats' knees, the mesenchymal stem cells retain enough iron to provide a strong MRI signal.
The new labeling technique alleviates the risks of contamination introduced when cells are labeled via manipulations in a laboratory dish—a major regulatory concern, said Daldrup-Link—as well as of a substantial loss of the delicate cells due to their extensive manipulation. It also allows for the immediate transfer of cells from a patient's bone marrow to the site of that patient's own knee injury.
That makes the technique useful in an autologous transplantation procedure, in which cells are extracted from a patient for the purpose of being delivered to another site during the same surgery. Jason Dragoo, MD, associate professor of orthopaedic surgery at the medical school and head team physician for the Stanford football program, plans to initiate a clinical trial this autumn whereby patients in need of knee repair will be treated with mesenchymal stem cells taken from their own bone marrow.
At Dragoo's request, Daldrup-Link's team began seeking a way of avoiding the delay and contamination risk associated with standard ways of labeling mesenchymal stem cells in a culture dish. "He asked us to find a way to label the cells without touching them," Daldrup-Link said. In anticipation of the upcoming clinical trial, one of Dragoo's trainees, medical student Malcolm Debaun, has taken up residence in the Daldrup-Link's lab in order to learn the technique in preparation for the upcoming clinical trial.
Daldrup-Link professes some amusement at the fact that an iron supplement can be used to track stem cells. "Often the simpler approaches are the ones that make it into the clinic," she said.
More information: Stanford has filed a provisional patent on the technique associated with this new use of ferumoxytol. The study was funded by the National Institutes of Health (grants 2R01AR054458-05, CCNE U54 CA119367, CCNE U54 CA151459 and R21CA138353A2).