Scientists transform non-beating human cells into heart-muscle cells
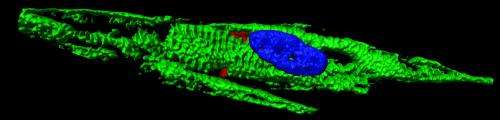
In the aftermath of a heart attack, cells within the region most affected shut down. They stop beating. And they become entombed in scar tissue. But now, scientists at the Gladstone Institutes have demonstrated that this damage need not be permanent—by finding a way to transform the class of cells that form human scar tissue into those that closely resemble beating heart cells.
Last year, these scientists transformed scar-forming heart cells, part of a class of cells known as fibroblasts, into beating heart-muscle cells in live mice. And in the latest issue of Stem Cell Reports, researchers in the laboratory of Gladstone Cardiovascular and Stem Cell Research Director Deepak Srivastava, MD, reveal that they have done the same to human cells in a petri dish.
"Fibroblasts make up about 50% of all cells in the heart and therefore represent a vast pool of cells that could one day be harnessed and reprogrammed to create new muscle," said Dr. Srivastava, who is also a professor at the University of California, San Francisco, with which Gladstone is affiliated. "Our findings here serve as a proof of concept that human fibroblasts can be reprogrammed successfully into beating heart cells."
In 2012, Dr. Srivastava and his team reported in the journal Nature that fibroblasts could be reprogrammed into beating heart cells by injecting just three genes, together known as GMT, into the hearts of live mice that had been damaged by a heart attack. They reasoned that the same three genes could have the same effect on human cells. But initial experiments on human fibroblasts from three sources—fetal heart cells, embryonic stem cells and neonatal skin cells—revealed that the GMT combination alone was not sufficient.
"When we injected GMT into each of the three types of human fibroblasts, nothing happened—they never transformed—so we went back to the drawing board to look for additional genes that would help initiate the transformation," said Gladstone Staff Scientist Ji-dong Fu, PhD, the study's lead author. "We narrowed our search to just 16 potential genes, which we then screened alongside GMT, in the hopes that we could find the right combination."
The research team began by injecting all candidate genes into the human fibroblasts. They then systematically removed each one to see which were necessary for reprogramming, and which were dispensable. In the end, the team found that injecting a cocktail of five genes—the 3-gene GMT mix plus the genes ESRRG and MESP1—were sufficient to reprogram the fibroblasts into heart-like cells. They then found that with the addition of two more genes, called MYOCD and ZFPM2, the transformation was even more complete. To help things along, the team initiated a chemical reaction known as the TGF-ß signaling pathway during the early stages of reprogramming, which further improved reprogramming success rates.
"While almost all the cells in our study exhibited at least a partial transformation, about 20% of them were capable of transmitting electrical signals—a key feature of beating heart cells," said Dr. Fu. "Clearly, there are some yet-to-be-determined barriers preventing a more complete transformation for many of the cells. For example, success rates might be improved by transforming the fibroblasts within living hearts rather than in a dish—something we also observed during our initial experiments in mice."
The immediate next steps are to test the five-gene cocktail in hearts of larger mammals, such as pigs. Eventually, the team hopes that a combination of small, drug-like molecules could be developed to replace the cocktail, offering a safer and easier method of delivery.
"With more than five million heart attack survivors in the United States, who have hearts that are no longer able to beat at full capacity, our findings—along with recently published findings from our colleagues—come at a critical time," added Dr. Srivastava. "We've now laid a solid foundation for developing a way to reverse the damage—something previously thought impossible—and changing the way that doctors may treat heart attacks in the future."
More information: Stem Cell Reports, Fu et al.: "Direct Reprogramming of Human Fibroblasts Toward a Cardiomyocyte-Like State." dx.doi.org/10.1016/j.stemcr.2013.07.005













