Research yields first detailed view of morphing Parkinson's protein
(Medical Xpress)—Researchers have taken detailed images and measurements of the morphing structure of a brain protein thought to play a role in Parkinson's disease, information that could aid the development of medications to treat the condition.
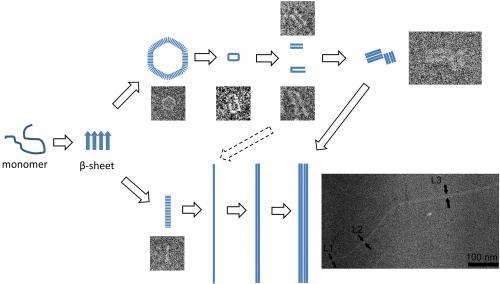
The protein, called alpha synuclein (pronounced sine-yoo-cline), ordinarily exists in a globular shape. However, the protein morphs into harmful structures known as amyloid fibrils, which are linked to protein molecules that form in the brains of patients with neurodegenerative diseases.
"The abnormal protein formation characterizes a considerable number of human diseases, such as Alzheimer's, Parkinson's and Huntington's diseases and type II diabetes," said Lia Stanciu, an associate professor of materials engineering at Purdue University.
Until now, the transition from globular to fibrils had not been captured and measured.
Researchers incubated the protein in a laboratory and then used an electron microscope and a technique called cryoelectron microscopy to snap thousands of pictures over 24 hours, capturing its changing shape. The protein was frozen at specific time intervals with liquid nitrogen.
Findings reveal that the protein morphs from its globular shape into "protofibril" strands that assemble into pore-like rings. These rings then open up, forming pairs of protofibrils that assemble into fibrils through hydrogen bonds.
"We found a correlation between proto?brils in these rings and the ?brils, for the ?rst time to our knowledge, by measuring their true sizes and visualizing the aggregation steps," Stanciu said. "A better understanding of the mechanism yields fresh insight into the pathogenesis of amyloid-related diseases and may provide us the opportunity to develop additional therapeutic strategies."
Parkinson's disease affects 1 percent to 2 percent of people older than 60, and an increase in its prevalence is anticipated in coming decades.
The findings were detailed in a research paper appearing in the June issue of the Biophysical Journal. The paper was authored by doctoral student Hangyu Zhang; former postdoctoral research associate Amy Griggs; Jean-Christophe Rochet, an associate professor of medicinal chemistry and molecular pharmacology; and Stanciu.
The researchers caused the protein to morph into fibrils by exposing it to copper, mimicking what happens when people are exposed to lead and other heavy metals. The contaminants interfere with the protein, changing the oxidation states of ions in its structure.